Abstract
A 77-year-old Japanese woman presented with asymptomatic abdominal lymphadenopathy. Soluble interleukin-2 receptor (sIL2R) and angiotensin-converting enzyme (ACE) levels were elevated, and a pathological examination of lymph-node biopsies revealed non-caseating granulomas, which was consistent with sarcoidosis. Fluorodeoxyglucose-positron emission tomography did not show a clear accumulation in the mediastinal lymph-nodes or heart. Five months later, she presented with acute progressive heart failure that was refractory to conventional treatment. Her sIL2R and ACE levels decreased spontaneously over time, without steroid treatment. Autopsy findings revealed non-caseating granulomas. Cardiac sarcoidosis presenting as acute, progressive, treatment-refractory heart failure is rare. Steroid therapy after the resolution of inflammation did not affect the clinical outcome.
Keywords: cardiac sarcoidosis, sarcoidosis activity, soluble interleukin-2 receptor (sIL2R), angiotensin converting enzyme (ACE)
Introduction
Sarcoidosis is a multi-system inflammatory disease characterized by non-caseating granulomas (1). Sarcoidosis generally affects the thoracic lymph nodes, lung, eyes, skin, and heart; intra-abdominal sarcoidosis and abdominal lymph node involvement are rare (2,3). The clinical manifestations of cardiac sarcoidosis are variable. Although cardiac sarcoidosis causes significant morbidity and mortality, it is difficult to recognize disease activity, characterize its severity, or predict the outcome (4,5). Rarely, cardiac sarcoidosis presents as acute progressive heart failure, that is refractory to conventional treatment (6,7).
Certain diagnostic markers are useful for evaluating the sarcoidosis activity. However, the association between the clinical course of cardiac sarcoidosis and the level of serum soluble interleukin-2 receptor (sIL2R) and angiotensin-converting enzyme (ACE), which have been reported to reflect sarcoidosis activity, remains unclear (8-10).
We herein report a case of sarcoidosis that presented with abdominal lymphadenopathy and high levels of sIL2R and ACE. Cardiac sarcoidosis was identified later, after these inflammatory markers had decreased. The patients' clinical course subsequently deteriorated rapidly despite aggressive therapy.
Case Report
A 77-year-old Japanese woman presented with asymptomatic abdominal lymphadenopathy, revealed on abdominal ultrasound. A blood examination demonstrated elevated levels of sIL2R (2,910 U/mL) and ACE (57.9 IU/L). Chest X-ray showed a cardiothoracic ratio of 44% (Fig. 1). Electrocardiography revealed normal sinus rhythm and non-specific T wave abnormalities (Fig. 2). Computed tomography (CT) revealed lymphadenopathy surrounding the hepatic duct and hepatic artery (Fig. 1). Fluorodeoxyglucose-positron emission tomography (FDG-PET) revealed a slight accumulation within the mediastinal lymph nodes. No FDG accumulation was observed within the heart or abdominal region. Echocardiography demonstrated good left ventricular (LV) wall motion. An open lymph node biopsy was performed six months later (day 0, Fig. 3). A pathological examination revealed non-caseating granulomas consistent with sarcoidosis. (Fig. 1D). The patient denied cardiovascular symptoms, hence a detailed cardiovascular examination was not performed.
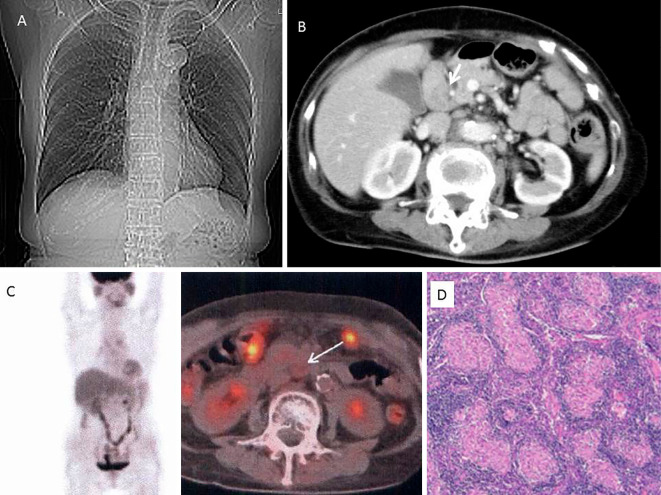
Figure 1.
A: Chest X-ray showed a cardiothoracic ratio of 44%. B: Extensive lymph adenopathy (white arrow) was observed around the hepatic duct and hepatic artery C: FDG-PET showed a minimal FDG accumulation in the right lower para-bronchial lymph node. No accumulation was observed within the abdominal lymph nodes (white arrow). D: A pathological examination of the abdominal lymph nodes revealed non-caseating granulomas.
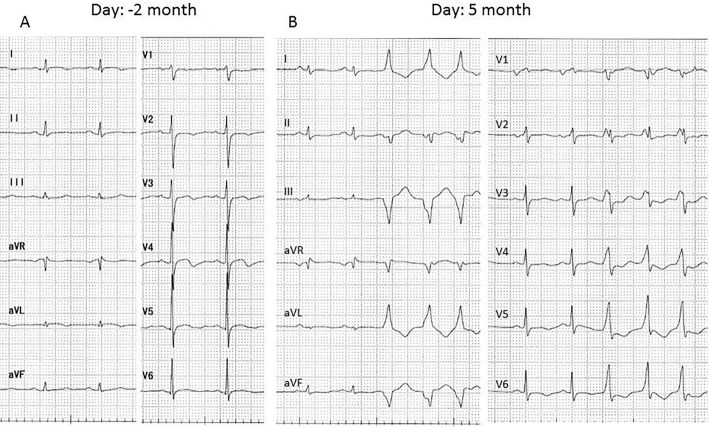
Figure 2.
A: Electrocardiography 2 months prior to the lymph node biopsy revealed normal sinus rhythm and T wave abnormalities. B: Electrocardiography recorded upon admission for heart failure revealed normal sinus rhythm and non-sustained monomorphic ventricular tachycardia.
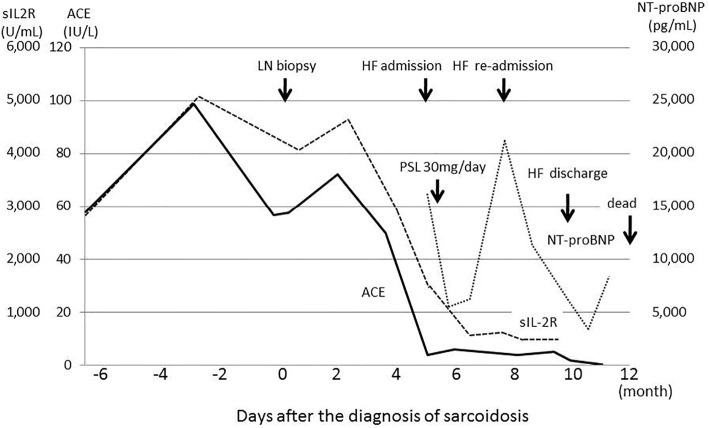
Figure 3.
Serum levels of sIL2R, ACE and NT-proBNP over time. sIL2R and ACE levels were elevated at the time of the abdominal lymph node biopsy. The serum levels subsequently decreased spontaneously, without steroid therapy. At the onset of heart failure symptoms, sIL2R was 1,300 U/mL, and ACE was 3.8 IU/L. NT-proBNP was continuously elevated after the onset of heart failure, despite aggressive therapy.
Five months after the initial diagnosis of sarcoidosis, she was admitted to our hospital with a chief complaint of dyspnea of several days duration. Until this time, her condition had been stable, no electrocardiography or chest imaging had been performed since her previous admission. During the present admission, she was diagnosed with heart failure (HF). A physical examination demonstrated a blood pressure of 94/62 mmHg and a heart rate of 95 bpm in sinus rhythm, although non-sustained ventricular tachycardia (VT) was observed (Fig. 2). Her height was 153 cm and weight 45 kg. The cervical, axillary, and inguinal lymph nodes were not palpable. On a cardiac examination, a Levine III/VI systolic murmur was auscultated at the left sternal border at the level of the 4th rib, and trace lower extremity edema was observed. A blood examination demonstrated elevated serum creatinine (1.14 mg/dL), HbA1c (6.3%), and C-reactive protein (CRP) (0.9 mg/dL). sIL2R was elevated to 1,300 U/mL and N-terminal prohormone of brain natriuretic peptide (NT-proBNP) was elevated to 16,981 pg/mL. Serum ACE was normal at 3.8 IU/L (Fig. 3). Serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT) and blood urea nitrogen (BUN) were normal at 35 IU/L, 14 IU/L and 19.2 mg/dL, respectively. Chest X-ray revealed cardiomegaly with blunting of the bilateral costophrenic angles (Fig. 4A). Echocardiography revealed diffuse LV hypokinesis and akinesis of the LV posterior wall without basal interventricular septum thinning (Fig. 4B). The LV diastolic dimension was 44 mm, and the LV ejection fraction was 22%. Coronary angiography showed no stenotic lesions. A myocardial biopsy from the right ventricular septum did not show non-caseating granulomas. Thallium scintigraphy revealed an abnormal fixed defect in the lateral region (Fig. 4C). Gallium scintigraphy showed normal accumulation (Fig. 4D). During her hospitalization, she developed an advanced atrio-ventricular block and a biventricular cardioverter defibrillator (CRT-D) was implanted. She was diagnosed with cardiac sarcoidosis and treated with 30 mg/day of prednisolone. She was also started on standard medical therapy for HF.
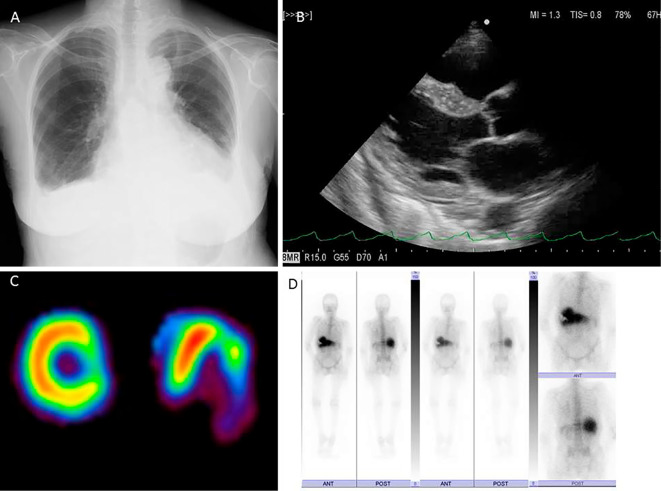
Figure 4.
A: Chest X-ray showed cardiomegaly and blunting of the bi-lateral costophrenic angles. B: Echocardiography demonstrated diffuse left ventricular (LV) hypo-kinesis and LV posterior/postero-lateral wall a-kinesis without basal inter-ventricular septum thinning. C: Adenosine stress thallium scintigraphy demonstrated a fixed defect in the lateral region. D: Gallium scintigraphy showed no pathological accumulation.
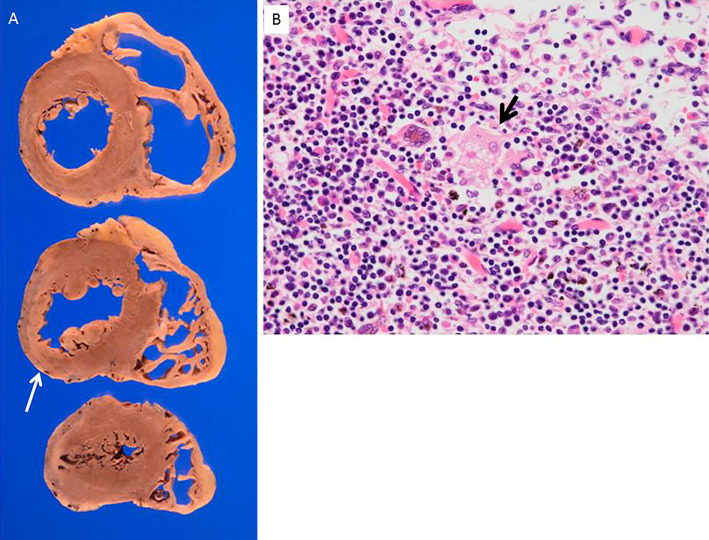
Upon discharge from the hospital, the patient was continued on prednisolone 10 mg/day. However, despite intensive medical therapy and CRT-D pacing, she remained New York Heart Association (NHYA) stage III, and her NT-proBNP remained elevated at 6,221 pg/mL. The level of sIL2R had decreased to 411 U/mL and the ACE level was stable at 6 IU/L. Both serum markers had decreased before the initiation of steroid therapy. One month later, she presented with orthopnea and worsening HF. In addition, the implantable cardioverter defibrillator (ICD) had discharged in order to terminate VT. After the in-hospital optimization of medical therapy, she was discharged on amiodarone 200 mg/day, prednisolone 12 mg/day, carvedilol 25 mg/day, azosemide 30 mg/day, spironolactone 25 mg/day and enarapril 10 mg/day. She remained NYHA stage III at discharge. After discharge, during resting activities at home, she developed several arrhythmias, including two episodes of VT that were terminated by CRT-D anti-tachycardia pacing or DC shock. Twelve months after the diagnosis of sarcoidosis, she collapsed at home. Electrocardiography (ECG) monitoring by emergency medical services revealed a-systole. Despite advanced cardiac life support, she was unable to be resuscitated. No episodes of VT/VF or ICD discharge had been recorded by the CRT-D device. An autopsy examination (Fig. 5) revealed thinning of the infero-posterior-lateral LV wall, that was related to the fixed defect region previously observed on thallium scintigraphy. However, non-caseating granulomas were not observed. Myocardial fibrosis and lymphocyte infiltration on the epicardial side of the left ventricular myocardium were also observed. Non-caseating granulomas were not observed in the systemic lymph nodes. However, asteroid bodies were observed in the mediastinal and right hilar lymph nodes.
Figure 5.
A: Pathological findings revealed wall thinning of the lateral, inferior, and posterior walls. Histological findings induced fibrosis and lymph cells infiltration, primarily beneath the epicardium. No non-caseating granulomas were observed. B Asteroid bodies (black arrow) were observed in the mediastinal and right hilar lymph nodes.
Discussion
Sarcoidosis is a systemic, inflammatory granulomatous disease characterized by non-caseating granulomas. Thoracic manifestations of the disease are common, but involvement of the abdominal lymph nodes, liver, or spleen is rare (2,3). Warshauer et al. reported that abdominal lymphadenopathy was found in 31% of sarcoidosis patients, and marked involvement was noted in only 10% (2). In the present case, abdominal lymphadenopathy was identified incidentally as an initial finding and diagnosed as sarcoidosis on a biopsy. At the time of the diagnosis, cardiac symptoms and reduced wall motion were not observed, although an extensive cardiac examination was not performed. No other organs were involved based on the CT and FDG-PET imaging findings. However, the patient subsequently developed acute progressive refractory heart failure and arrhythmias due to cardiac sarcoidosis. Cardiac sarcoidosis with abdominal lymphadenopathy as an initial symptom is rare, and it can be the difficult to diagnose cardiac involvement in early stages of disease.
In this case, both ACE and sIL2R were elevated at the time of the diagnosis, (57.9 IU/L and 2,819 U/mL, respectively) and remained elevated thereafter (>50 IU/L and >3,000 U/mL respectively) (Fig. 3). It has been reported that sIL2R and ACE levels are correlated with the sarcoidosis activity and severity. In particular, sIL2R is reported to be a marker of the severity of pulmonary sarcoidosis and extra-pulmonary involvement (8-10). However, no previous reports have investigated the clinical usefulness of these markers or their relation to clinical outcomes in cardiac sarcoidosis. In our case, sIL2R and ACE levels were extremely elevated at the time of the initial diagnosis, and prior to the development of cardiac symptoms. FDG-PET did not show a clear accumulation in the mediastinal lymph nodes or heart, despite the patient having high levels of sIL2R and ACE. As previously reported, corticosteroid therapy appears to attenuate sarcoidosis related inflammation. However, both serum markers decreased spontaneously, and had largely normalized by the time prednisolone therapy was initiated. Corticosteroid therapy has long-term benefits, particularly in the prevention of heart failure and preservation of the LV systolic function (11). In this case, cardiac involvement was not detected by CT or FDG-PET at the time of the diagnosis, when ACE and sIL2R values were elevated, and prednisolone therapy was started after the ACE and sIL2R levels had decreased. This timing may help explain the patient's corticosteroid-refractory cardiac sarcoidosis.
In order to prevent deleterious outcomes, it may be necessary to start corticosteroid therapy early in patients with cardiac sarcoidosis. In particular, in patients with elevated levels of ACE or sIL2R, a careful cardiac evaluation and follow- up are needed.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors thank Dr. Yoshikazu Yazaki (Department of Cardiovascular Division, Saku Central Hospital Advanced Care Center) for his clinical comments.
References
- 1. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. New Engl J Med 357: 2153-2165, 2007. [DOI] [PubMed] [Google Scholar]
- 2. Warshauer DM, Dumbleton SA, Molina PL, et al. Abdominal CT findings in sarcoidosis: radiologic and clinical correlation. Radiology 192: 93-98, 1994. [DOI] [PubMed] [Google Scholar]
- 3. MacArthur KL, Forouhar F, Wu YH. Intra-abdominal complications of sarcoidosis. J Formos Med Assoc 109: 484-492, 2010. [DOI] [PubMed] [Google Scholar]
- 4. Tavora F, Cresswell N, Li L, Ripple M, Solomon C, Burke A. Comparison of necropsy findings in patients with sarcoidosis dying suddenly from cardiac sarcoidosis versus suddenly from other cause. Am J Cardiol 104: 571-577, 2009. [DOI] [PubMed] [Google Scholar]
- 5. Otsuka K, Terasaki F, Eishi Y, et al. Cardiac sarcoidosis underlies idiopathic dilated cardiomyopathy. Importance of mediastinal lymphadenopathy in differential diagnosis. Circ J 71: 1937-1941, 2007. [DOI] [PubMed] [Google Scholar]
- 6. Sukkgizaki Y, Tanaka H, Imanishi J, et al. Isolated primary cardiac sarcoidosis presenting as acute heart failure. Intern Med 52: 71-74, 2013. [DOI] [PubMed] [Google Scholar]
- 7. Chau EMC, Fan KYY, Chow WH. Cardiac sarcoidosis: a potentially fatal but treatable form of infiltrative heart disease. Hong Kong Med J 12: 65-67, 2006. [PubMed] [Google Scholar]
- 8. Gungor S, Ozeker F, Yalcinsoy M, et al. Conventional markers in determination of activity of sarcoidosis. Int Immunopharmacol 25: 174-179, 2015. [DOI] [PubMed] [Google Scholar]
- 9. Grustters JC, Fellrath JM, Mulder L, et al. Serum soluble interleukin-2 receptor measurement in patients with sarcoidosis. Chest 124: 186-195, 2003. [DOI] [PubMed] [Google Scholar]
- 10. Miyoshi S, Hamada H, Kadowaki T, et al. Comparative evaluation of serum markers in pulmonary sarcoidosis. Chest 137: 1391-1397, 2010. [DOI] [PubMed] [Google Scholar]
- 11. Nagai T, Nagano N, Sugano Y, et al. Effect of corticosteroid therapy on long-term clinical outcome and left ventricular function in patients with cardiac sarcoidosis. Circ J 79: 1593-1600, 2015. [DOI] [PubMed] [Google Scholar]