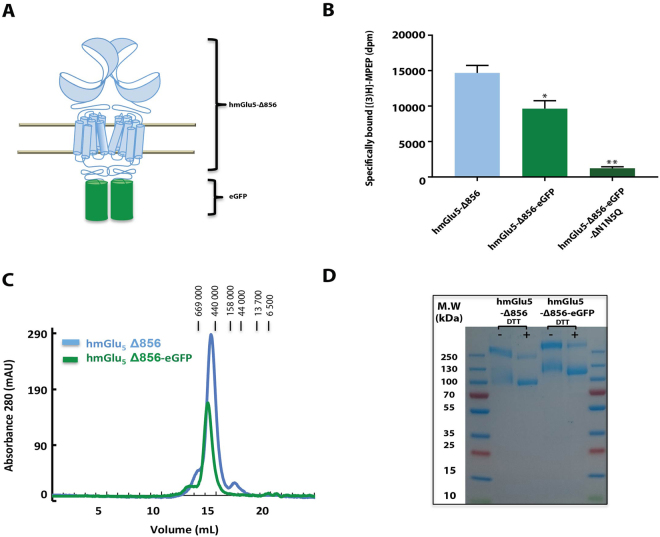
Figure 4.
Purification of the dimer mGlu5 -∆856 produced in SF9 cells. (A) Cartoon representation of the dimer human mGlu5 receptor (light blue) with the fusion protein eGFP (green) replacing the c-terminal domain at position 856. (B) Quantification of unpurified mGlu5 receptor using [3H]-MPEP binding assay, measured for mGlu5-Δ856, mGlu5-Δ856-eGFP and mGlu5-Δ856-eGFP (N1-N5). Dunnett’s test as part of one-way ANOVA was used for comparison with mGlu5-Δ856 set as reference level. (C) Size exclusion chromatography profile for both constructs, mGlu5-Δ856 (Ve = 14.5 mL) and mGlu5-Δ856-eGFP (Ve = 14.2 mL) using superpose 6 increase. (D) Coomassie-blue stained SDS-PAGE gel of mGlu5-Δ856 and mGlu5-Δ856-eGFP. The gel shown is representative of three independent experiments.