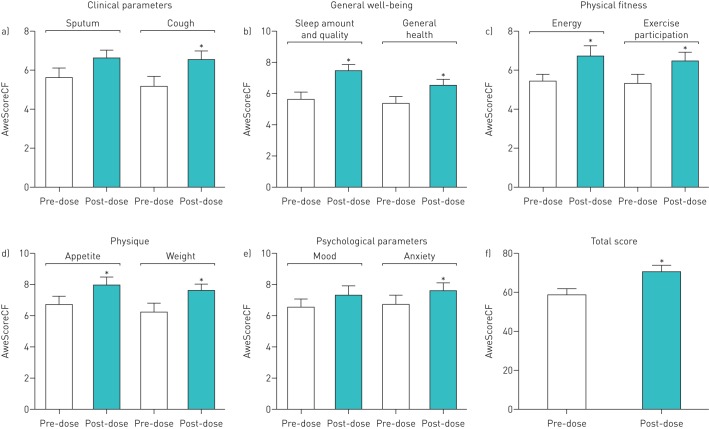
FIGURE 4.
Results of the AweScoreCF questionnaire completed by 23 adult cystic fibrosis (CF) patients 1 month before and 3 months after initiating ivacaftor–lumacaftor therapy: a) clinical parameters, b) general well-being, c) physical fitness, d) physique, e) psychological parameters and f) total score. Data are presented as mean±sem of a paired t-test. *: p<0.05. In eight out of 10 subdomains changes resulted in statistically significant improvement of quality of life and well-being of the 23 adult CF patients; a positive trend was observed in the subdomains sputum and mood.