Abstract
Background
Stroke is associated with an increased risk of dementia; however, the impact of stroke on cognition has been found to be variable, such that stroke survivors can show decline, remain stable, or revert to baseline cognitive functioning. Knowing the natural history of cognitive impairment after stroke is important for intervention. The aim of this systematic review is to investigate the longitudinal course of cognitive function in stroke survivors.
Methods and Results
Three electronic databases (Medline, Embase, PsycINFO) were searched using OvidSP from inception to July 15, 2016. Longitudinal studies with ≥2 time points of cognitive assessment after stroke were included. In total, 5952 articles were retrieved and 14 were included. There was a trend toward significant deterioration in cognitive test scores in stroke survivors (8 studies). Cognitive stability (3 studies) and improvement (3 studies) were also demonstrated, although follow‐up time tended to be shorter in these studies. Variables associated with impairment included age, ethnicity, premorbid cognitive performance, depression, stroke location, and history of previous stroke. Associations with APOE*E4 (apolipoprotein E with the E4 allele) allele status and sex were mixed.
Conclusions
Stroke is associated with an increased risk of cognitive decline, but cognitive decline is not a consequence. Factors associated with decline, such as sociodemographic status, health‐related comorbidity, stroke history, and clinical features could be used in models to predict future risk of dementia after stroke. A risk model approach could identify patients at greatest risk for timely intervention to reduce the frequency or delay the onset of poststroke cognitive impairment and dementia.
Keywords: cognition, cognitive impairment, dementia, risk factors/global assessment, stroke
Subject Categories: Cognitive Impairment, Risk Factors
Clinical Perspective
What Is New?
Cognitive outcome following a stroke is dependent on sociodemographic, health, and stroke‐related risk factors and the timing of cognitive assessment.
What Are the Clinical Implications?
Poststroke patients need to have their cognitive function followed up over time to ensure that cognitive decline is noted early.
Known risk factors associated with poststroke cognitive decline could be incorporated into risk scores to ensure timely detection of poststroke cognitive decline.
Introduction
Stroke is the second most common cause of acquired cognitive impairment, which predisposes patients toward institutionalization, disability, increased mortality, and poorer quality of life.1, 2, 3 With an aging population and a decline in mortality after stroke,4 the rates of poststroke cognitive impairment will increase. Despite being as common as other neurological deficits, such as motor and sensory, cognitive impairment is often overlooked in the follow‐up of stroke survivors unless they have progressed to dementia.5 This may well be because these patients are able to maintain some level of personal independence despite poor cognitive recovery.6
It has been found that stroke survivors may show no cognitive deficits or may decline, initially decline and then improve, remain stable, or progress to dementia over time.7, 8 Mixed findings may be related to differences in the cognitive tests used and test timing, history of previous stroke, stroke location, large‐ and small‐vessel disease, population sample (clinical versus population based), ethnicity, and the presence of neurodegenerative pathology.9 Nevertheless, it is also possible that the initial poststroke cognitive state may reflect prestroke cognitive decline10 or delirium.11 There is a drive toward detecting long‐term cognitive outcomes after stroke to explore prevention; however, a preferred testing strategy is lacking, making cross‐study comparison difficult.12
The aim of this systematic review was to assess the longitudinal pattern of cognitive function in stroke survivors and to determine those factors associated with change over time. Recognizing the natural history of cognitive impairment after stroke is vital for informing early treatment and preventative strategies.
Methods
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure. This material can be made available by the corresponding author on reasonable request.
Search Strategy and Selection Criteria
This systematic review was undertaken in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) statement.13 The review was registered with PROSPERO (CRD42014015018). Three electronic databases—Medline, Embase, and PsycINFO—were searched using OvidSP from inception to July 15, 2016; searches were restricted to human studies and articles published in English. Predefined and Boolean search terms were used, including stroke, (cognit* or neuropsych*), and (progress* or longitudinal or decline or prospective). Longitudinal studies with ≥2 time points of cognitive assessment after stroke were included. No distinction was made regarding the sampling framework (clinic, hospital, or population based), the number of strokes, or the timing of cognitive assessments after stroke or cognitive battery used. Studies in which baseline and subsequent incident stroke cases found at follow‐up were analyzed together were included. No distinction was made regarding the mechanism of stroke, and studies were not excluded if stroke was not confirmed using neuroimaging. All participants were adults who were aged ≥50 years and free from dementia. Randomized controlled trials and cognitive rehabilitation studies were excluded. Studies in which the only outcome was a diagnosis of dementia were excluded because this was the subject of a previous review.14 Studies were excluded if change in cognitive function over time in the stroke group was not reported (ie, studies that only compared cognitive outcomes in stroke patients versus controls were excluded). Studies were also excluded if they reported percentages of decline rather than actual test scores or did not report statistical comparison of change in cognitive performance over time.
Four authors (O.A., E.Y.H.T., E.G., and S.L.H.) independently searched the article titles and abstracts. If the article could not be rejected with certainty based on title or abstract alone, then the full text of the article was obtained. Discrepancies between authors were resolved by consensus, and if this was not possible, then they were resolved by a third author (B.C.M.S.). Four authors (O.A., E.Y.H.T., E.G., and S.L.H.) carried out evaluation of full‐text articles. Consensus or a third author resolved disagreements. The reference lists of the full‐text articles and any relevant reviews were searched for potential eligible references.
Data Extraction
Data extracted included study design, sample size, demographic characteristics, inclusion or exclusion criteria, definition of stroke, cognitive test battery, and results. Three authors (E.Y.H.T., S.L.H., and E.G.) extracted data independently, and any discrepancies were resolved through consensus or discussion with a third author (B.C.M.S.). Because of the heterogeneity in the study design (eg, variation in follow‐up time, cognitive test battery used), a meta‐analysis was not possible.
Results
Figure shows the results of the electronic search and article‐selection process. The search identified 9365 articles, of which 3413 were duplicates and thus removed. After reviewing titles and abstracts, 238 articles were retained for full‐text review. The main reasons for exclusion were that the study population age was <50 years, cognitive measures were not reported, and a cross‐sectional design was used. Fourteen articles met the inclusion criteria.
Figure 1.
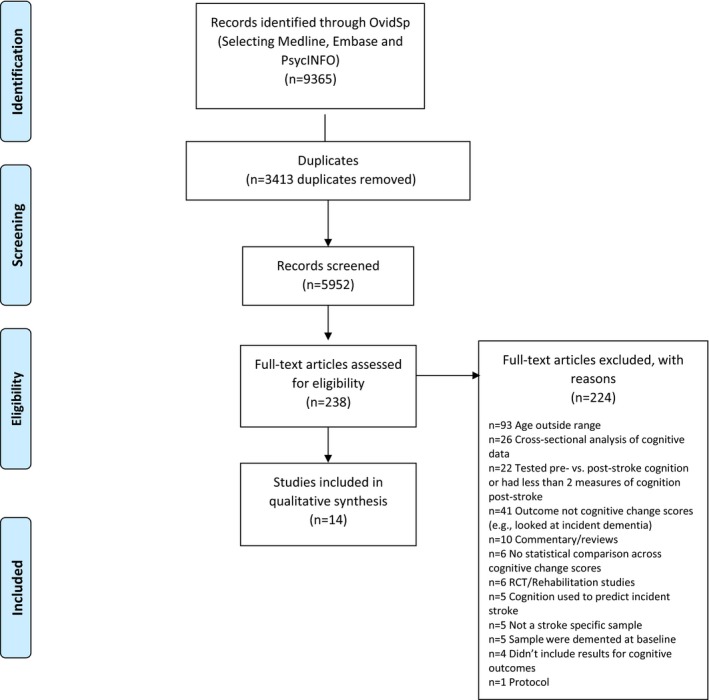
Flow diagram showing article selection. RCT indicates randomized controlled trial.
Study Characteristics
Characteristics of the included studies and detailed cognitive outcomes are shown in Tables S1 and S2, respectively. The number of participants at baseline ranged from 5015 to 1187.16 Follow‐up ranged from 3 weeks17 to 6 years.15, 18, 19 The cohorts included stroke‐specific populations17, 20, 21, 22, 23 and population‐based studies.15, 16, 18, 19, 24, 25, 26, 27, 28 The majority of studies were conducted in the United States (n=4),16, 18, 25, 26 followed by the United Kingdom (n=2),20, 27 Israel (n=2),22, 23 the Netherlands (n=2),15, 24 Germany (n=1),19 India (n=1),28 Norway (n=1),21 and Japan (n=1).17 Stroke case ascertainment included hospital‐based diagnosis,20, 21, 22, 23, 27 self‐report,15, 25 general practitioner records,19 self‐report confirmed through medical records and/or expert review,16, 18, 24, 26, 28 and not specified.17 Only 2 studies used magnetic resonance imaging data.22, 23
Cognitive Assessments
The Mini Mental State Examination (MMSE; total,16, 17, 21, 24, 28 subtests scores,26 and modified MMSE [3MSE]25) was the most commonly used cognitive assessment. Other batteries for assessing global cognitive function included the Montreal Cognitive Assessment (MoCA),22, 23 the Cambridge Cognitive Assessment (CAMCOG),27 the revised CAMCOG (CAMCOG‐R),20 and the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS).21 Domain‐specific cognitive tests included the Auditory Verbal Learning Test (immediate and delayed recalled),24 the Alphabet Coding Task,15, 24 East Boston Test,16 the Symbol Digits Modalities Test,16 subtests of the neuropsychological test battery of the Consortium to Establish a Registry for Alzheimer's Disease,19 2 subsets of Raven's Coloured Progressive Matrices,15 and the word‐list delayed recall of the Spanish and English Verbal Learning Test.25 The computerized neuropsychological assessment NeuroTrax was used by 2 studies but in the same cohort.22, 23
Study Sampling Framework
The majority of cohorts were population based (n=8),15, 16, 18, 19, 24, 25, 26, 28 although 5 studies were hospital based,17, 20, 21, 22, 23 and the sampling framework was unclear in 1 study.27 The studies demonstrating cognitive decline tended to be population‐based cohorts with longer follow‐up (3 years28 to 6 years15, 18). Studies demonstrating cognitive recovery were all hospital‐based cohorts with shorter follow‐up (3 weeks17 to 13 months21). Cognitive outcomes were also reported in separate studies from the same population in 2 cohorts: a hospital‐based cohort (Tel Aviv Brain Acute Stroke Cohort)22, 23 and a population‐based cohort (Longitudinal Aging Study Amsterdam).15, 24
Cognitive Function After Stroke
The impact of stroke on cognitive function over time was mixed as shown in the Table.
Table 1.
Characteristics and Cognitive Findings From Included Studies (n=14)
| Author | N | Sampling Framework | Follow‐up | Cognitive Assessment | Key Findings | Risk Variables |
|---|---|---|---|---|---|---|
| Decline | ||||||
| Comijs, 200915 | 50 T1, 90 T2, 84 T3 | Population‐based cohort | Maximum 6 y | MMSE, RCPM, ACT, AVLT (immediate and delayed recall) |
Significant decline in memory (immediate and delayed recall) and information processing speed No significant change in global cognitive function |
N/A |
| Rajan, 201416 | 1187 | Population‐based cohort | Mean 4.2 y (SD 3.9) | East Boston Test (immediate and delayed story recall), Symbol Digits Modalities Test, MMSE (total and orientation scores) | Significant decline in global cognitive function | Increased risk of decline among black patients compared with white patients (all tests) |
| Ghosal, 201428 | 283 | Population‐based cohort | Maximum 3 y | MMSE (Bengali version) | Significant decline in global cognitive function | Global impairment more common in women, higher age of onset of stroke, and people with higher depression scores |
| Levine, 201325 | 151 | Population‐based cohort | Mean poststroke follow‐up of 3.6 y for women and 3.4 y for men | Modified MMSE (3MSE), Word‐List Delayed Recall of the Spanish and English Verbal Learning Test (SEVLT) |
No significant change in global cognitive function or verbal memory No significant overall sex differences |
No effect of systolic blood pressure on global cognition |
| Reitz, 200626 | 97 | Population‐based cohort | Maximum 5 y | Orientation (MMSE items), Boston Naming Test, Controlled Word Association Test, category naming, Boston Diagnostic Aphasia Evaluation (Complex Ideational Material and Phrase Repetition), WAIS‐R similarities subtest, nonverbal Identities and Oddities subtest of the Mattis Dementia Rating Scale, Rosen Drawing Test, Benton (matching), Benton Visual Retention Test and the Selective Reminding Test |
Significant decline in memory No significant change in abstract/visuospatial or language |
Significant decline in memory in men and abstract/visuospatial in APOE*E4‐negative patients |
| Ben Assayag, 201522 | 298 | Hospital‐based cohort | Maximum 2 y | MoCA and computerized global cognitive score (including memory, executive functions, visuospatial perception, verbal function, attention and motor skills) | Significant decline in global cognition in those taking longer to complete the TUG | Multivariable model: Age ≥75 y, TUG score >12 s at 6 mo after stroke, MoCA score 6 mo after stroke |
| Tene, 201623 | 306 | Hospital‐based cohort | Maximum 2 y | As above | Significant decline in global cognition, memory, executive functioning and visuospatial in those with higher admission and six‐month GDS scores; attention also declined in those who had higher GDS scores at 6 months | Multivariable model: MoCA score at hospital admission, age ≥75 y, GDS score ≥6 (admission and 6 mo after stroke) |
| Toole, 200418 | 5364 | Population‐based cohort | Maximum 6 y | 3MS | Significant decline in global cognitive function | Left‐hemisphere (highest decline) and right‐hemisphere strokes |
| Stability | ||||||
| Kohler, 201219 | 3214 | Population‐based cohort | Maximum 4.5 y | CERAD verbal fluency and recall (immediate and delayed) tasks | No significant change in verbal fluency, immediate or delayed recall | Not reported |
| Rowan, 200727 | 126 | Unclear | Maximum 27 months | CAMCOG and the Cognitive Drug Research computerized battery | N/A | No significant decline in global cognitive function when stratified by homocysteine levels |
| Dik, 200024 | 53 | Population‐based cohort | Mean 3.1 y (SD 0.2) | MMSE, AVLT (immediate and delayed), Coding Task (information processing speed) | No significant change in global cognition, memory, or information processing speed (adjusted models) | Lowered risk for global cognitive decline for APOE*E4 carriers (not significant) |
| Recovery | ||||||
| Leeds, 200120 | 83 | Hospital‐based cohort | Maximum 3 months | CAMCOG‐R, Weigl color form sorting test, Raven's matrices | Significant improvement in global and executive function | Depression influenced executive function and CAMCOG‐R scores |
| Wagle, 201021 | 104 | Hospital‐based cohort | Mean 408.4 d (SD 41.2) | RBANS and MMSE |
Significant improvement in visuospatial/constructional, delayed memory and global cognition (RBANS only) No significant change in global cognition (MMSE), immediate memory, language and attention |
Multivariable model: Presence of APOE*E4, prestroke cognitive reduction, previous stroke, and neurological impairment |
| Suzuki, 201317 | 57 | Hospital‐based cohort | Maximum 3 wk | MMSE | Significant improvement in global cognitive function | Not reported |
ACT indicates Alphabet Coding Task; AVLT, Auditory Verbal Learning Test; CAMCOG, Cambridge Cognitive Assessment; CAMCOG‐R, Cambridge Cognitive Assessment (Revised); CERAD, Consortium to Establish a Register for Alzheimer's Disease; GDS, Geriatric Depression Score; MMSE, Mini Mental State Examination; MoCA, Montreal Cognitive Assessment; N/A, not assessed; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; RCPM, Raven's Colored Progressive Matrices; SEVLT, Spanish and English Verbal Learning Test; T, time point; 3MSE, Modified Mini Mental State Examination; TUG, Timed Up and Go; WAIS‐R, Wechsler Adult Intelligence Scale–Revised.
Global cognitive function
Most studies (n=12) included a measure of global cognitive function. Of these, 3 studies16, 18, 28 reported significant decline (follow‐up: 3–6 years), 3 studies15, 21, 25 reported no change (follow‐up: 13 months to 6 years), and 3 studies17, 20, 21 reported significant improvement (follow‐up: 3 weeks to 13 months) over time. In stratified analyses (4 studies), it was found (1) that although there was no significant decline in global function (3MSE score), over 3 years of follow‐up in the whole sample with sex‐stratified analysis, both men and women showed significant changes in 3MSE errors (worse in men than women, but no significant sex differences)25; (2) that stroke patients with slower physical performance, measured using the Timed Up and Go test, performed significantly worse on a computerized global cognitive test battery compared with stroke patients with faster physical performance (follow‐up duration: 2 years)22; (3) that stroke patients with comorbid depression performed significantly worse on global cognitive scores compared with stroke patients without depression (follow‐up duration: 2 years)23; and (4) that there was no significant difference in CAMCOG scores when stroke patients were stratified by homocysteine levels (follow‐up duration: 27 months).27
Memory
Six studies included tests of memory.15, 19, 21, 24, 25, 26 Of these, 2 reported significant decline including impairments in immediate and delayed recall (follow‐up: 6 years)15 and visual memory (follow‐up: 5 years).29 Four studies reported no significant change in measures of verbal memory (follow‐up: >3 years),25 immediate memory (follow‐up: 13 months),21 or immediate and delayed recall (follow‐up: 3.1–4.5 years).19, 24 One study found an improvement in delayed memory over 13 months of follow‐up.21 In stratified analyses, 1 study reported significant decline in memory for those with higher Geriatric Depression Scale (GDS) scores.23 One study reported a significant decline in memory over 5‐year follow‐up and was also found to be strongest for men compared with women.26
Nonmemory
Five studies included nonmemory tests.15, 19, 21, 24, 26 One study reported a significant decline in information processing speed over 6 years of follow‐up.15 Three studies reported no changes in nonmemory performance including measures of abstract reasoning,26 visuospatial ability,26 verbal fluency,19 attention,21 information processing speed,24 and language performance21, 26 (follow‐up: 13 months to 5 years). One study reported significant improvement in executive function over 3 months follow‐up,20 and another reported significant improvements in visuospatial/constructional performance over 13 months follow‐up.21 In stratified analysis, 1 study reported significant declines in executive function and visuospatial domains for those with higher admission and 6 month GDS scores; attention also declined in those who had higher GDS scores at 6 months.23 A further study reported a significant decline in abstract/visuospatial scores in patients who were negative versus positive for APOE*E4 (apolipoprotein E with the E4 allele).26
Risk Factors for Poststroke Cognitive Decline
Risk factors for cognitive impairment included ethnicity (greater risk in black patients compared with white patients),16 depression,23, 28 increased age,22, 23, 28 sex (mixed results25, 28), APOE*E4 status (mixed findings21, 24, 26), poorer cognitive performance after stroke,22 stroke location (left and right hemisphere),18 and a previous history of stroke.21 Findings for sex were mixed: 1 study found no sex differences,25 1 found a greater risk of global impairment in women,28 and another found a greater risk of decline in memory in men.26 In 1 study, systolic blood pressure was not associated with global cognitive function >3 years after stroke in either men or women.25
Discussion
In this systematic review, the effect of stroke on longitudinal changes in cognitive function before a diagnosis of dementia was found to be mixed depending on the cognitive domain tested and methodology factors (eg, follow‐up time). Furthermore, risk factors traditionally associated with cognitive decline, including APOE*E4 status24, 26 and systolic blood pressure level,25 did not have the same expected effect in stroke‐specific samples. Accurate identification of stroke survivors at highest risk of cognitive decline is important and could be used to identify people for early intervention and participation in clinical trials.
Regarding global cognitive function, the majority of studies reported decline,16, 18, 22, 23, 25, 28 whereas 5 reported no change.15, 21, 24, 25, 27 In contrast, 3 studies utilizing different cognitive batteries (MMSE17, CAMCOG‐R,20 and RBANS21) reported recovery. Recovery as measured by MMSE and CAMCOG‐R could be due to a combination of the small sample size of the study (57 patients17 and 83 patients20) and short follow‐up (3 weeks17 and up to 3 months20). Furthermore, the study using the RBANS had a similarly small sample size (n=104) and short follow‐up (13 months) and restricted the study population to those fulfilling requirements for rehabilitation, meaning that the study would have excluded the more severe strokes and thus potentially those at greater risk of cognitive decline.21 When stroke survivors were followed up for a longer period of time (eg, ≥3 years), a significant decline in global cognition was reported.16, 18, 28 Given that age is the biggest risk factor for incident dementia,30 future studies could look at whether or not age of onset of stroke and disease duration determines the longitudinal cognitive trajectory after stroke. This would involve comparing stroke cohorts that had younger onset with an older stroke population and following their cognition over time. This approach could help describe global cognitive recovery in all stroke populations and assess who is at greatest risk of cognitive nonrecovery.
When assessing domain‐specific function the results were mixed. Studies identified improvements in executive function,20 visuospatial/constructional performance,21 and memory (delayed)21 over 3 to 13 months of follow‐up. When participants were followed for longer periods (eg, ≥3 years), studies reported significant decline in memory (in some studies15, 26 but not all18, 23, 28) and no significant change in abstract/visuospatial performance.26 Findings were mixed for information‐processing speed, with 1 study reporting no significant change over 3 years of follow‐up24 but another reporting significant decline over 6 years of follow‐up.15 Two studies also found no significant change in language performance.21, 26 These mixed findings could be driven by varying sample size and heterogeneity in study design as well as by differences in the length of follow‐up and medical treatments. These results highlight the importance of testing different cognitive domains in stroke survivors and the need to develop a consensus cognitive battery to allow cross‐study comparison. Further work could assess the effect of stroke severity, subtypes, or locations on cognitive domains and factors that could assist in cognitive recovery.
Across studies, risk factors for cognitive decline included demographic factors (age, sex, ethnicity), neuropsychiatric symptoms (depression), disease‐related comorbidity (previous stroke), poorer baseline cognitive tests, genetic factors (ie, APOE*E4 status), function (balance and gait), and the nature of the stroke itself (stroke location). Factors such as arterial hypertension and the number of cerebral infarcts have been shown to be prognostic variables of cognitive deterioration.31 However, not all factors were consistently observed to increase risk, including sex and APOE*E4 status. With regard to sex, global impairment was found to be more common in women in 1 study,28 which is comparable to existing literature.32 In contrast, in another study, although there was no significant sex difference, global cognitive decline was found to be more severe in men.25 However, this study was performed only in older Mexican Americans and may reflect only the relative risk found for this ethnicity. When specific domains were tested, when stratified by sex, men were found to show significant decline in memory.26 However, the sample size for men was much smaller than that for women (n=27 versus n=70, respectively), which raises issues of statistical power. Regarding APOE*E4 status, 1 study (n=19 who were APOE genotype 4/−) found a significant association between stroke and decline in abstract/visuospatial performance in those without the APOE*E4 allele.26 Another (n=27 APOE*E4 positive)24 found that stroke patients without the APOE*E4 allele showed faster decline on global cognition, although this was not statistically significant and a synergistic effect was not observed.24 In contrast, yet another study found that being an APOE*E4 carrier was predictive of cognitive impairment at 13 months after stroke, but again, the sample size was small (n=25).21 Given the inconsistency of these results with small sample sizes, further research is warranted to identify whether APOE*E4 carriers with a history of stroke are at a higher risk of future poststroke cognitive impairment.
A number of risk scores have been developed to predict dementia in whole populations, with many using modifiable risk factors (eg, vascular risk factors33, 34) with the hope that modifying these factors could alter cognitive trajectory. A risk model approach could be used in stroke populations, incorporating some of these variables identified in this review to predict poststroke dementia.35 A number of risk scores have been developed recently to predict poststroke dementia (3 months after stroke, area under the curve: 0.74)36 and cognitive impairment (6 months after stroke, area under the curve: 0.83).37 Our review, however, shows that cognitive decline seems to become more apparent over a longer follow‐up period, and thus new models could be developed to predict poststroke cognitive impairment and dementia over longer time periods. Currently there are no specific biomarkers that can help discriminate between those at risk and those with better prognosis.38 There is evidence, however, of a strong relationship between inflammation markers and cognitive performance,39 and this will need further evaluation before being used in potential risk models. Although neuroimaging variables were used in the cognitive impairment model,37 evidence shows that data from magnetic resonance imaging do not significantly improve prediction in all‐cause dementia models.40 Similarly, there may be less focus on incorporating vascular risk factors into these models because results from a recent clinical trial found that intensively managing vascular risk factors in stroke survivors did not alter cognition after 2 years.41
Strengths and Limitations
This study has a number of strengths. We performed a systematic search of all studies focusing on older aged samples. Furthermore, we did not restrict our search by cognitive domain. This is important in stroke samples, for which overall cognitive improvement may be explained by significant improvements in some nonmemory domains but individuals may still show persisting memory deficits. We also ensured that the included studies had statistical comparisons of change in cognitive test scores over time. Nevertheless, there are limitations. Only studies in English were included, and the majority of studies were in white populations. Consequently, the results may not extrapolate to nonwhite samples. Studies were also excluded if the sample baseline age was ≤50 years because stroke before age 50 is uncommon, and these patients may have a different risk profile than the older population.
Conclusions
Cognitive outcomes after stroke can be variable, and standardized assessment tools together with recommended time intervals for testing are needed. Determinants of poststroke cognitive decline are important to clarify, particularly if these patients are different from the nonstroke population; interventions may need to be tailored specifically to stroke survivors. A number of risk factors for cognitive decline, particularly in global functioning, in stroke survivors have been found, such as age, sex, stroke location, and medical comorbidities (depression), and could be incorporated into a risk tool to identify stroke survivors at highest risk of cognitive decline over short and long durations of follow‐up.
Sources of Funding
Tang is supported by a National Institute for Health Research (NIHR) Doctoral Research Fellowship (DRF‐2015‐08‐006). Robinson is supported by an NIHR professorship (NIHR‐RP‐011‐043) and an NIHR Senior Investigator award. Siervo is supported by a Medical Research Council Grant (MR/N007921/1).
Disclosures
None.
Supporting information
Table S1. Study Characteristics
Table S2. Baseline and Follow‐up Cognitive Measures
Acknowledgments
We would like to thank Andy Bryant for providing statistical expertise in the critical appraisal of the included studies.
(J Am Heart Assoc. 2018;7:e006443 DOI: 10.1161/JAHA.117.006443.)29335318
References
- 1. O'Brien JT, Erkinjuntti T, Reisberg B, Roman G, Sawada T, Pantoni L, Bowler JV, Ballard C, DeCarli C, Gorelick PB, Rockwood K, Burns A, Gauthier S, DeKosky ST. Vascular cognitive impairment. Lancet Neurol. 2003;2:89–98. [DOI] [PubMed] [Google Scholar]
- 2. Ankolekar S, Renton C, Sare G, Ellender S, Sprigg N, Wardlaw JM, Bath PM. Relationship between poststroke cognition, baseline factors, and functional outcome: data from “efficacy of nitric oxide in stroke” trial. J Stroke Cerebrovasc Dis. 2014;23:1821–1829. [DOI] [PubMed] [Google Scholar]
- 3. Leys D, Hénon H, Mackowiak‐Cordoliani MA, Pasquier F. Poststroke dementia. Lancet Neurol. 2005;4:752–759. [DOI] [PubMed] [Google Scholar]
- 4. Rothwell PM, Coull AJ, Giles MF, Howard SC, Silver LE, Bull LM, Gutnikov SA, Edwards P, Mant D, Sackley CM, Farmer A, Sandercock PA, Dennis MS, Warlow CP, Bamford JM, Anslow P. Change in stroke incidence, mortality, case‐fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study). Lancet. 2004;363:1925–1933. [DOI] [PubMed] [Google Scholar]
- 5. Jacova C, Pearce LA, Costello R, McClure LA, Holliday SL, Hart RG, Benavente OR. Cognitive impairment in lacunar strokes: the SPS3 trial. Ann Neurol. 2012;72:351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prevo AJ, Dijkman MM, Le Fevre FA. Impairment and disability in patients with a severe ischemic cerebral infarction at admission to the rehabilitation center and six months after stroke [Dutch]. Ned Tijdschr Geneeskd. 1998;142:637–640. [PubMed] [Google Scholar]
- 7. Rasquin SM, Lodder J, Verhey FR. Predictors of reversible mild cognitive impairment after stroke: a 2‐year follow‐up study. J Neurol Sci. 2005;229–230:21–25. [DOI] [PubMed] [Google Scholar]
- 8. Ballard C, Rowan E, Stephens S, Kalaria R, Kenny RA. Prospective follow‐up study between 3 and 15 months after stroke: improvements and decline in cognitive function among dementia‐free stroke survivors >75 years of age. Stroke. 2003;34:2440–2444. [DOI] [PubMed] [Google Scholar]
- 9. Kalaria RN, Akinyemi R, Ihara M. Stroke injury, cognitive impairment and vascular dementia. Biochem Biophys Acta. 2016;1862:915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre‐stroke and post‐stroke dementia: a systematic review and meta‐analysis. Lancet Neurol. 2009;8:1006–1018. [DOI] [PubMed] [Google Scholar]
- 11. Shi Q, Presutti R, Selchen D, Saposnik G. Delirium in acute stroke: a systematic review and meta‐analysis. Stroke. 2012;43:645–649. [DOI] [PubMed] [Google Scholar]
- 12. Lees R, Selvarajah J, Fenton C, Pendlebury ST, Langhorne P, Stott DJ, Quinn TJ. Test accuracy of cognitive screening tests for diagnosis of dementia and multidomain cognitive impairment in stroke. Stroke. 2014;45:3008–3018. [DOI] [PubMed] [Google Scholar]
- 13. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–e34. [DOI] [PubMed] [Google Scholar]
- 14. Savva GM, Stephan BC; Alzheimer's Society Vascular Dementia Systematic Review G . Epidemiological studies of the effect of stroke on incident dementia: A systematic review. Stroke. 2010;41:e41–e46. [DOI] [PubMed] [Google Scholar]
- 15. Comijs HC, Kriegsman DM, Dik MG, Deeg DJ, Jonker C, Stalman WA. Somatic chronic diseases and 6‐year change in cognitive functioning among older persons. Arch Gerontol Geriatr. 2009;48:191–196. [DOI] [PubMed] [Google Scholar]
- 16. Rajan KB, Aggarwal NT, Wilson RS, Everson‐Rose SA, Evans DA. Association of cognitive functioning, incident stroke, and mortality in older adults. Stroke. 2014;45:2563–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suzuki M, Sugimura Y, Yamada S, Omori Y, Miyamoto M, Yamamoto J. Predicting recovery of cognitive function soon after stroke: differential modeling of logarithmic and linear regression. PLoS One. 2013;8:e53488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Toole JF, Bhadelia R, Williamson JD, Veltkamp R. Progressive cognitive impairment after stroke. J Stroke Cerebrovasc Dis. 2004;13:99–103. [DOI] [PubMed] [Google Scholar]
- 19. Kohler M, Kliegel M, Kaduszkiewicz H, Bachmann C, Wiese B, Bickel H, Mosch E, Weyerer S, Werle J, Fuchs A, Pentzek M, Leicht H, Konig HH, Luppa M, Riedel‐Heller S, Jessen F, Maier W, Scherer M, Wagner M. Effect of cardiovascular and metabolic disease on cognitive test performance and cognitive change in older adults. J Am Geriatr Soc. 2012;60:1286–1291. [DOI] [PubMed] [Google Scholar]
- 20. Leeds L, Meara RJ, Woods R, Hobson JP. A comparison of the new executive functioning domains of the CAMCOG‐R with existing tests of executive function in elderly stroke survivors. Age Ageing. 2001;30:251–254. [DOI] [PubMed] [Google Scholar]
- 21. Wagle J, Farner L, Flekkoy K, Wyller TB, Sandvik L, Eiklid KL, Fure B, Stensrod B, Engedal K. Cognitive impairment and the role of the apoe epsilon4‐allele after stroke–a 13 months follow‐up study. Int J Geriatr Psychiatry. 2010;25:833–842. [DOI] [PubMed] [Google Scholar]
- 22. Ben Assayag E, Shenhar‐Tsarfaty S, Korczyn AD, Kliper E, Hallevi H, Shopin L, Auriel E, Giladi N, Mike A, Halevy A, Weiss A, Mirelman A, Bornstein NM, Hausdorff JM. Gait measures as predictors of poststroke cognitive function: evidence from the TABASCO study. Stroke. 2015;46:1077–1083. [DOI] [PubMed] [Google Scholar]
- 23. Tene O, Shenhar‐Tsarfaty S, Korczyn AD, Kliper E, Hallevi H, Shopin L, Auriel E, Mike A, Bornstein NM, Assayag EB. Depressive symptoms following stroke and transient ischemic attack: is it time for a more intensive treatment approach? Results from the TABASCO cohort study. J Clin Psychiatry. 2016;77:673–680. [DOI] [PubMed] [Google Scholar]
- 24. Dik MG, Deeg DJ, Bouter LM, Corder EH, Kok A, Jonker C. Stroke and apolipoprotein E epsilon4 are independent risk factors for cognitive decline: a population‐based study. Stroke. 2000;31:2431–2436. [DOI] [PubMed] [Google Scholar]
- 25. Levine DA, Haan MN, Langa KM, Morgenstern LB, Neuhaus J, Lee A, Lisabeth LD. Impact of gender and blood pressure on poststroke cognitive decline among older Latinos. J Stroke Cerebrovasc Dis. 2013;22:1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reitz C, Luchsinger JA, Tang MX, Manly J, Mayeux R. Stroke and memory performance in elderly persons without dementia. Arch Neurol. 2006;63:571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rowan EN, Dickinson HO, Stephens S, Ballard C, Kalaria R, Kenny RA. Homocysteine and post‐stroke cognitive decline. Age Ageing. 2007;36:339–343. [DOI] [PubMed] [Google Scholar]
- 28. Ghosal MK, Burman P, Singh V, Das S, Paul N, Ray BK, Hazra A, Banerjee TK, Basu A, Chaudhuri A, Das SK. Correlates of functional outcome among stroke survivors in a developing country—a prospective community‐based study from India. J Stroke Cerebrovasc Dis. 2014;23:2614–2621. [DOI] [PubMed] [Google Scholar]
- 29. Reitz C, Bos MJ, Hofman A, Koudstaal PJ, Breteler MM. Prestroke cognitive performance, incident stroke, and risk of dementia: the Rotterdam Study. Stroke. 2008;39:36–41. [DOI] [PubMed] [Google Scholar]
- 30. Fratiglioni L, Launer LJ, Andersen K, Breteler MM, Copeland JR, Dartigues JF, Lobo A, Martinez‐Lage J, Soininen H, Hofman A. Incidence of dementia and major subtypes in Europe: a collaborative study of population‐based cohorts. Neurologic diseases in the Elderly Research Group. Neurology. 2000;54:S10–S15. [PubMed] [Google Scholar]
- 31. Gomez‐Viera N, Martin‐Labrador M, Guevara‐Ferrer M, Jimenez‐Paneque R, Amaro‐Hernandez A, Munoz‐Navarro S. Prognostic factors of cognitive deterioration in patients with cerebral infarcts [Spanish]. Revista de Neurologia. 2002;34:223–231. [PubMed] [Google Scholar]
- 32. Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tang EY, Harrison SL, Errington L, Gordon MF, Visser PJ, Novak G, Dufouil C, Brayne C, Robinson L, Launer LJ, Stephan BC. Current developments in dementia risk prediction modelling: an updated systematic review. PLoS One. 2015;10:e0136181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stephan BCM, Kurth T, Matthews FE, Brayne C, Dufouil C. Dementia risk prediction in the population: are screening models accurate? Nat Rev Neurol. 2010;6:318–326. [DOI] [PubMed] [Google Scholar]
- 35. Tang EYH, Robinson L, Stephan BCM. Risk prediction models for post‐stroke dementia. Geriatrics. 2017;2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lim JS, Oh MS, Lee JH, Jung S, Kim C, Jang MU, Lee SH, Kim YJ, Kim Y, Park J, Kang Y, Yu KH, Lee BC. Prediction of post‐stroke dementia using ninds‐csn 5‐minute neuropsychology protocol in acute stroke. Int Psychogeriatr. 2017;29:777–784. [DOI] [PubMed] [Google Scholar]
- 37. Kandiah N, Chander RJ, Lin X, Ng A, Poh YY, Cheong CY, Cenina AR, Assam PN. Cognitive impairment after mild stroke: development and validation of the SIGNAL2 risk score. J Alzheimers Dis. 2016;49:1169–1177. [DOI] [PubMed] [Google Scholar]
- 38. Mijajlovic MD, Pavlovic A, Brainin M, Heiss WD, Quinn TJ, Ihle‐Hansen HB, Hermann DM, Assayag EB, Richard E, Thiel A, Kliper E, Shin YI, Kim YH, Choi S, Jung S, Lee YB, Sinanovic O, Levine DA, Schlesinger I, Mead G, Milosevic V, Leys D, Hagberg G, Ursin MH, Teuschl Y, Prokopenko S, Mozheyko E, Bezdenezhnykh A, Matz K, Aleksic V, Muresanu D, Korczyn AD, Bornstein NM. Post‐stroke dementia—a comprehensive review. BMC Med. 2017;15:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kliper E, Bashat DB, Bornstein NM, Shenhar‐Tsarfaty S, Hallevi H, Auriel E, Shopin L, Bloch S, Berliner S, Giladi N, Goldbourt U, Shapira I, Korczyn AD, Assayag EB. Cognitive decline after stroke: relation to inflammatory biomarkers and hippocampal volume. Stroke. 2013;44:1433–1435. [DOI] [PubMed] [Google Scholar]
- 40. Stephan BC, Tzourio C, Auriacombe S, Amieva H, Dufouil C, Alperovitch A, Kurth T. Usefulness of data from magnetic resonance imaging to improve prediction of dementia: population based cohort study. BMJ. 2015;350:h2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bath PM, Scutt P, Blackburn DJ, Ankolekar S, Krishnan K, Ballard C, Burns A, Mant J, Passmore P, Pocock S, Reckless J, Sprigg N, Stewart R, Wardlaw JM, Ford GA. Intensive versus Guideline Blood Pressure and Lipid Lowering in Patients with Previous Stroke: Main Results from the Pilot ‘Prevention of Decline in Cognition after Stroke Trial’ (PODCAST) Randomised Controlled Trial. PLoS One. 2017;12:e0164608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Study Characteristics
Table S2. Baseline and Follow‐up Cognitive Measures


