Abstract
Background
Mitral regurgitation is a heterogeneous disease. Determining which patients derive optimal outcomes from transcatheter edge‐to‐edge mitral valve repair (TMVR) remains challenging. We sought to determine whether baseline mitral valve anatomic characteristics are predictive of left atrial pressure (LAP) changes during TMVR with MitraClip.
Methods and Results
Consecutive patients with severe mitral regurgitation undergoing TMVR (n=112) underwent continuous intraprocedural LAP monitoring and retrospective echocardiographic analysis for specific mitral anatomic characteristics. Procedural success (optimal LAP reduction) was defined as ≥40% reduction in left atrial V‐wave pressure compared with baseline. Echocardiographic predictors of optimal LAP reduction and increased postprocedure mean diastolic gradient were evaluated. Mean age was 79±14 years, and 36 patients (32%) were women. Primary, mixed, and secondary mitral regurgitation were present in 78 patients (70%), 22 patients (20%), and 12 patients (10%), respectively. Baseline mean LAP and V‐wave were 22±6 and 38±13 mm Hg; after TMVR, these decreased to 19±5 and 27±10 mm Hg, respectively (P<0.0001 for both). Independent predictors of optimal LAP reduction were the presence of a flail scallop, mitral regurgitation localized to a single scallop, and high‐quality 3‐dimensional echocardiographic imaging. Independent predictors of elevated postprocedure mean diastolic gradient were elevated preprocedure mean diastolic gradient, mitral annular calcification, and implantation of multiple clips.
Conclusions
Mitral valve pathoanatomic features, including a flail leaflet and single jet, are predictive of optimal LAP reduction with TMVR. High‐quality 3‐dimensional imaging may help select patients with the highest likelihood of optimal hemodynamic results with TMVR. These data expand current knowledge about patient selection for TMVR and deserve further study in larger cohorts.
Keywords: echocardiography, left atrial pressure, MitraClip, mitral regurgitation
Subject Categories: Echocardiography, Valvular Heart Disease, Catheter-Based Coronary and Valvular Interventions
Clinical Perspective
What Is New?
In a consecutive cohort referred for transcatheter edge‐to‐edge mitral valve repair, the presence of a flail scallop, a single regurgitant jet, and optimal 3‐dimensional echocardiographic image quality predict optimal left atrial pressure reduction postprocedure. An elevated preprocedure mean diastolic gradient, mitral annular calcification, and implantation of multiple clips predict a postprocedure mean diastolic gradient of >5 mm Hg.
What Are the Clinical Implications?
These data expand current knowledge in patient selection for transcatheter edge‐to‐edge mitral valve repair and help inform medical decision making in patients under consideration for transcatheter edge‐to‐edge repair versus novel alternative transcatheter mitral valve therapies or conventional open mitral valve surgery.
Introduction
Mitral regurgitation (MR) is among the most commonly encountered valve disease lesions, becoming increasingly prevalent with age.1 Data indicate that patients are likely underreferred for surgical therapy, often on the basis of advanced age and increased comorbidities, which confer increased surgical risk.2 Transcatheter mitral valve repair (TMVR) using the MitraClip (Abbott, Santa Clara, CA) is a novel minimally invasive and clinically approved technique for edge‐to‐edge repair of primary MR. Compared with open surgical repair, TMVR is associated with similar improvements in left ventricular remodeling and long‐term survival, with reduced risk of perioperative morbidity and mortality,3, 4, 5, 6, 7 making it an attractive therapeutic option for patients with severe MR at high surgical risk.
In practice, MR is a heterogeneous disease, and it remains challenging to determine which patients are most likely to derive optimal outcomes with TMVR. Proposed anatomic criteria for MitraClip candidacy have included thresholds of flail gap and flail width, minimal mitral valve area, and minimal calcification in the leaflet grasping area, although these lack supporting data.8 To date, it is unknown how pathologic features, such as specific leaflet abnormalities (eg, flail segment or posterior leaflet shortening), jet location, number of regurgitant jets, and mitral annular calcification, affect procedural success in patients undergoing TMVR. It is also unclear what baseline echocardiographic features predict the development of significant mitral stenosis after creation of the double orifice and increased diastolic pressure gradient across the mitral valve.
Grading the severity of MR by echocardiography after implantation remains challenging, because there are frequently multiple jets and eccentric jets, and the visual appearance is dependent on loading conditions that are dynamic during the procedure. We have recently shown that an alternative measure of procedural success, left atrial V‐wave reduction, is associated with greater improvements in 6‐minute walk distance at 30 days after TMVR.9 The present study aimed to determine whether baseline mitral valve anatomic characteristics are predictive of left atrial V‐wave pressure reduction or increased mean diastolic gradient after TMVR with MitraClip.
Methods
The data that support the findings of this study will be made available to other researchers on reasonable request from the corresponding author for the purpose of reproducing the results. This study was approved by the Mayo Clinic Institutional Review Board. Informed consent was waived given the minimal risk and retrospective nature of the study. Consecutive patients with severe MR undergoing TMVR with the MitraClip between May 21, 2014 and September 30, 2016 were included. All patients were evaluated by a Heart Team, including a cardiologist and a cardiac surgeon with expertise in mitral valve surgery, and determined to be high risk for surgery.
Left Atrial Pressure Analysis
The MitraClip procedure was performed under general anesthesia with systemic arterial pressure monitoring using standardized techniques.10 All patients underwent continuous left atrial pressure (LAP) monitoring during TMVR. Briefly, a 4F fluid‐filled multipurpose catheter was inserted into the left atrium alongside the MitraClip steerable guide using previously described methods.11 This allowed for continuous LAP recording throughout the procedure. Continuous pressure tracings were digitally recorded for offline storage and review using proprietary software (CathCoding; Mayo Clinic, Rochester, MN). LAP was measured offline following the procedure at time points that were immediately before and immediately after clip deployment to minimize the impact of procedural changes in preload and afterload on the LAP measurement. Procedural success (optimal LAP reduction) was defined as ≥40% reduction in left atrial V‐wave compared with baseline.9
Mitral Valve Anatomic Scoring
Preprocedural and intraprocedural transesophageal echocardiograms were retrospectively reviewed by experienced echocardiographers (J.J.T., J.F.M., V.T.N., and S.V.P.) blinded to the procedural result and LAP measurements. Specific mitral valve anatomic characteristics included the following: presence/absence of flail leaflet, flail width >15 mm, flail gap >10 mm, location of the dominant mitral regurgitant jet (A2/P2 versus other location as assessed by Carpentier's classification), the presence and location of any secondary regurgitant jets, posterior mitral leaflet grasping length, presence/absence of mitral annular calcification, presence/absence of leaflet calcification, commissural prolapse involving the dominant regurgitant jet,12 maximum width of the dominant regurgitant jet (vena contracta), baseline mitral valve mean diastolic gradient, postprocedure mitral mean diastolic gradient, the presence/absence of posterior mitral leaflet cleftlike indentations, and the presence of redundant mitral leaflets with extensive prolapse involving ≥50% of mitral leaflet tissue. MR severity was graded using an integrated multiparametric approach in accordance with current American Society of Echocardiography guidelines.13 Left ventricular ejection fraction, left ventricular end‐diastolic dimension, left atrial volume index, and right ventricular systolic pressure were obtained from the preprocedure transthoracic echocardiogram.
Echocardiographic Image Quality Assessment
Two‐ and 3‐dimensional (3D) imaging quality was graded on a scale of excellent, good, and suboptimal, taking into account image spatial and temporal (frame/volume rate) resolution and the ability to visualize the device and mitral valve leaflets. “Excellent” image quality was characterized by excellent temporal and spatial resolution, resulting in precise visualization of the device and corresponding mitral valve leaflet pathology. When there was minor degradation of temporal and/or spatial resolution that did not significantly interfere with diagnostic confidence, image quality was graded as “good.” Image degradation was frequently related to acoustic shadowing from the device or guide, suboptimal esophageal imaging windows, or, at times, inadequate optimization of 3D volume size or gain. In patients with “suboptimal” image quality, there was significant degradation in spatial or temporal resolution that reduced diagnostic confidence in determining the mitral leaflet pathology, the location of the device, or the spatial relationship between the 2.
Statistical Analysis
Variables were compared between groups using unpaired Student t tests with an a priori significance defined as P<0.05. Categorical variables were compared using the χ2 test or Fisher exact test. The univariate analysis was performed using χ2 analysis for categorical variables or logistic regression for continuous variables to determine correlates of optimal LAP reduction (defined as left atrial V‐wave reduction of ≥40% compared with baseline)9 or increased mean mitral diastolic gradient postprocedure (>5 mm Hg). All preprocedural echocardiographic parameters were considered for inclusion in the multivariable analysis. Parameters with a P<0.05 by univariate analysis were included as candidate variables in the multivariable analysis using nominal logistic regression. Multivariable predictors of optimal LAP reduction and increased mean diastolic gradient postprocedure were used to create a composite risk score for each end point. Points were assigned to each variable in direct proportion to the adjusted risk ratio, and the total for each patient was summed to create the composite score. Interobserver variability in anatomic scoring and assessment of echocardiographic imaging quality were evaluated in a subset of 20 patients. Statistical analysis was performed using JMP (SAS software version 12.0; SAS Institute Inc, Cary, NC).
Results
Two patients with functional mitral valve clefts, 2 patients with mitral valve perforations, and 3 patients in whom LAP was not measured postprocedure were excluded from the analysis; the remaining 112 patients were included.
Patient Characteristics and Postprocedure Outcomes
Baseline clinical characteristics are shown Table 1. Mean age was 79±14 years, and 32% of the cohort was women. There were high rates of concurrent comorbidities (mean Society for Thoracic Surgeons risk score, 10.1±5.9%), 49% had prior cardiac surgery, 24% had moderate or greater lung disease, 31% had prior myocardial infarction, and 71% had atrial fibrillation. Among the cohort of 112 patients, 78 (70%) had primary MR, 22 (20%) had a mixed mechanism, and 12 (11%) had a secondary (functional) MR.
Table 1.
Clinical Characteristics
| Parameter | Value |
|---|---|
| Age, y | 79±14 |
| Female sex | 36 (32) |
| Prior stroke or TIA | 18 (16) |
| Peripheral arterial disease | 25 (22) |
| Current smoker | 10 (9) |
| Diabetes mellitus | 24 (21) |
| Moderate or greater lung disease | 27 (24) |
| Prior myocardial infarction | 35 (31) |
| Atrial fibrillation | 80 (71) |
| NYHA functional class | |
| III | 80 (71) |
| IV | 32 (29) |
| STS mortality score, % | 10.1±5.9 |
| Prior cardiac surgery | 55 (49) |
Data are given as mean±SD or number (percentage). NYHA indicates New York Heart Association; STS, Society for Thoracic Surgeons; and TIA, transient ischemic attack.
Postprocedural MR reduction is outlined in Table 2. Two patients in the series experienced new pericardial effusion and cardiac tamponade intraprocedurally, and both cases were successfully treated with percutaneous drainage. Single leaflet detachment was noted in 1 patient intraprocedurally, and this was managed by implantation of 2 additional devices, with a good procedural result and stabilization of the partially detached clip. Follow‐up echocardiograms were available in 87 of 112 patients at a mean follow‐up of 43±24 days. MR grade was unchanged in 53 patients (61%), increased by 1 grade in 23 patients (26%), and increased by ≥2 grades in 11 patients (13%). Mean clinical follow‐up was 283±223 days, and mortality was 15% at 6 months and 25% at 1 year in patients with follow‐up data available. During follow‐up, repeated TMVR was performed in 5 patients (4%) and an open mitral valve surgery was performed in 9 patients (8%).
Table 2.
Echocardiographic and Procedural Variables According to LAP Reduction and Postprocedural MnG
| Parameter | All Patients (n=112) | LAP Reduction | MnG Postprocedure | ||||
|---|---|---|---|---|---|---|---|
| Optimal LAP Reduction (n=29) | Lower LAP Reduction (n=83) | P Value | MnG >5 mm Hg (n=19) | MnG ≤5 mm Hg (n=93) | P Value | ||
| LV EF, % | 51±14 | 51±15 | 52±13 | 0.82 | 54±14 | 51±14 | 0.40 |
| End‐diastolic diameter, mm | 56±8 | 57±9 | 56±8 | 0.75 | 54±8 | 57±8 | 0.17 |
| LAVI, mL/m2 | 65±21 | 62±16 | 67±22 | 0.16 | 58±15 | 67±21 | 0.05 |
| RVSP, mm Hg | 53±15 | 55±19 | 53±14 | 0.67 | 53±13 | 53±15 | 0.94 |
| Preprocedure mean diastolic gradient, mm Hga | 2±1 | 2±2 | 2±1 | 0.94 | 3±1 | 2±1 | 0.01 |
| Preprocedure MR severity | 0.09 | 0.53 | |||||
| Grade 3/4 | 18 (16) | 2 (7) | 16 (19) | 4 (21) | 14 (15) | ||
| Grade 4/4 | 94 (84) | 27 (93) | 67 (81) | 15 (79) | 79 (85) | ||
| Preprocedure ERO, cm2 | 0.45±0.15 | 0.50±0.16 | 0.43±0.15 | 0.05 | 0.38±0.11 | 0.46±0.16 | 0.02 |
| Preprocedure RV, mL | 68±21 | 76±22 | 66±20 | 0.06 | 62±12 | 70±22 | 0.06 |
| Postprocedure MR severity | 0.04 | 0.06 | |||||
| Grade 1/4 | 71 (63) | 23 (79) | 47 (57) | 7 (37) | 63 (68) | ||
| Grade 2/4 | 33 (29) | 6 (21) | 27 (33) | 9 (47) | 24 (26) | ||
| Grade 3/4 | 4 (4) | 0 (0) | 4 (5) | 2 (11) | 2 (2) | ||
| Grade 4/4 | 5 (4) | 0 (0) | 5 (6) | 1 (5) | 4 (4) | ||
| Flail scallop | 46 (41) | 19 (66) | 27 (33) | 0.002 | 7 (36) | 39 (42) | 0.80 |
| Flail gap >10 mm | 3 (3) | 2 (7) | 1 (1) | 0.13 | 0 (0) | 3 (3) | >0.99 |
| Flail width >15 mm | 9 (8) | 4 (14) | 5 (6) | 0.21 | 0 (0) | 9 (9) | 0.35 |
| Flail gap >10 mm or flail width >15 mm | 12 (11) | 6 (21) | 6 (7) | 0.06 | 0 (0) | 12 (13) | 0.21 |
| A2‐P2 jet origin | 73 (65) | 23 (79) | 50 (60) | 0.06 | 14 (74) | 59 (63) | 0.38 |
| Multiple regurgitant jets | 51 (46) | 10 (34) | 41 (49) | 0.16 | 13 (68) | 38 (41) | 0.03 |
| >1 Jet involving at least 2 scallops | 39 (35) | 5 (17) | 34 (41) | 0.02 | 11 (58) | 28 (30) | 0.02 |
| Maximum jet width, mm | 10±4 | 10±4 | 10±4 | 0.73 | 9±3 | 10±4 | 0.12 |
| PML length, mm | 15±4 | 15±4 | 14±3 | 0.11 | 14±3 | 15±4 | 0.34 |
| Commissural involvement | 6 (5) | 1 (4) | 5 (6) | 0.60 | 0 (0) | 6 (7) | 0.59 |
| Mitral annular calcification | 49 (44) | 14 (48) | 35 (42) | 0.57 | 15 (79) | 34 (37) | 0.0009 |
| Calcified mitral leaflets | 23 (21) | 3 (10) | 20 (24) | 0.10 | 6 (32) | 17 (18) | 0.22 |
| Redundant leaflets with ≥50% leaflet prolapse | 14 (13) | 4 (14) | 10 (12) | 0.81 | 1 (5) | 13 (14) | 0.46 |
| PML CLI | 54 (51) | 18 (64) | 36 (46) | 0.10 | 7 (47) | 47 (52) | 0.72 |
| 2D echo image quality | 0.32 | 0.35 | |||||
| Suboptimal | 2 (2) | 0 (0) | 2 (2) | 1 (5) | 1 (1) | ||
| Good | 62 (55) | 14 (48) | 48 (58) | 12 (63) | 50 (54) | ||
| Excellent | 48 (43) | 15 (52) | 33 (40) | 6 (32) | 42 (45) | ||
| 3D echo image quality | 0.04 | 0.75 | |||||
| Suboptimal | 23 (21) | 2 (7) | 21 (25) | 5 (26) | 18 (19) | ||
| Good | 60 (54) | 16 (55) | 44 (53) | 10 (53) | 50 (54) | ||
| Excellent | 29 (26) | 11 (38) | 18 (22) | 4 (21) | 25 (27) | ||
| Postprocedure mean diastolic gradient, mm Hg | 4±2 | 4±1 | 4±2 | 0.32 | 7±1 | 3±1 | <0.0001 |
| Postprocedure heart rate, bpm | 73±14 | 73±13 | 73±14 | 0.82 | 77±14 | 72±14 | 0.22 |
| >1 Clip deployed | 44 (40) | 11 (39) | 33 (41) | 0.89 | 13 (68) | 31 (34) | 0.006 |
| Postprocedure NYHA functional classb | 0.01 | 0.24 | |||||
| I | 54 (53) | 20 (77) | 34 (45) | 10 (59) | 44 (52) | ||
| II | 31 (30) | 4 (15) | 27 (36) | 2 (12) | 29 (34) | ||
| III | 15 (15) | 1 (4) | 14 (18) | 4 (24) | 11 (13) | ||
| IV | 2 (2) | 1 (4) | 1 (1) | 1 (6) | 1 (1) | ||
Values are displayed as number (percentage) or mean±SD. 2D indicates 2 dimensional; 3D, 3 dimensional; bpm, beats per minute; CLI, cleft‐like indentation; EF, ejection fraction; ERO, estimated regurgitant orifice; LAP, left atrial pressure; LAVI, left atrial volume index; LV, left ventricular; MnG, mean diastolic gradient; MR, mitral regurgitation; NYHA, New York Heart Association; PML, posterior mitral leaflet; RV, regurgitant volume; and RVSP, right ventricular systolic pressure.
Preprocedure MnG available in 55 patients.
Postprocedure NYHA class available in 102 patients.
Baseline Mitral Valve Anatomic Features
Baseline mitral valve anatomic features are summarized in Table 2. Although a flail scallop was frequently present (46/112 [41%]), flail gap >10 mm and flail width >15 mm were relatively uncommon (3% and 8%, respectively). Compared with patients without flail leaflet, those with a flail had larger estimated regurgitant orifice area (0.53±18 versus 0.39±0.10 cm2; P=0.0001) and regurgitant volume (77±24 versus 63±16 mL; P=0.002).
The dominant regurgitant jet was most frequently found at A2‐P2 (73/112 [65%]), and commissural involvement was infrequent (6/112 [5%]). Multiple regurgitant jets were present in 51 patients (46%), and in 39 patients (35%) these jets involved >1 scallop. The maximum jet width (vena contracta), frequently measured in the commissural view, was 10±4 mm. Mitral annular calcification was also common (49/112 [44%]), but the mitral leaflets were less commonly involved (23/112 [21%]). Extensive mitral leaflet prolapse, defined as prolapse of ≥50% of the mitral leaflet tissue with redundant‐appearing leaflets, was present in a minority of patients (14/112 [13%]). Two‐dimensional echocardiographic image quality was rated as good or excellent in most cases (110/112 [98%]). Three‐dimensional echocardiographic image quality was rated good or excellent quality in 89 of 112 patients (79%).
Predictors of Optimal LAP Reduction
At baseline, the left atrial mean pressure and V‐wave were 22±6 mm Hg and 38±13 mm Hg, respectively. After TMVR, these lowered to 19±5 mm Hg and 27±10 mm Hg, respectively (P<0.0001 for both). V‐wave reduction of at least 5 mm Hg occurred in 84 patients (74%) and at least 10 mm Hg in 56 patients (50%). Overall, the V‐wave decreased by 24±26% postprocedure, and 29 of 112 patients (26%) achieved optimal LAP V‐wave reduction of ≥40%. Preprocedure systolic blood pressure (114±16 versus 108±16 mm Hg; P=0.13), postprocedure systolic blood pressure (112±18 versus 110±14 mm Hg; P=0.51), and the preprocedure versus postprocedure change in systolic blood pressure (2±21 versus −1±14 mm Hg; P=0.49) were similar in those who did and did not have optimal LAP reduction.
Echocardiographic and anatomic variables according to LAP reduction are shown in Table 2. Patients with optimal LAP reduction were more likely to have a flail scallop (66% versus 33%; P=0.002), and there was a trend for increased prevalence of a flail gap >10 mm or flail width >15 mm (21% versus 7%; P=0.06). Patients with flail leaflet had a larger reduction in the V‐wave postprocedure compared with those without a flail leaflet (Figure 1). Among patients with flail leaflet, there was no significant difference in percentage reduction of the V‐wave in patients with flail gap >10 mm or flail width >15 mm (37±26% versus 31±27%; P=0.56). Those with optimal LAP reduction tended to be more likely to have an A2‐P2 jet origin (79% versus 60%; P=0.06). Multiple jets of regurgitation were present with similar frequency in those with versus without optimal LAP reduction (34% versus 49%; P=0.16). However, those with optimal LAP reduction were less likely to have >1 jet involving at least 2 scallops (17% versus 41%; P=0.02). Two‐dimensional echocardiography image quality was similar between those with and without optimal LAP reduction, but patients with optimal LAP reduction were more likely to have 3D image quality that was graded good or excellent (93% versus 75%; P=0.04).
Figure 1.

Left atrial V‐wave pressure reduction according to flail leaflet, number of jets, and 3‐dimensional (3D) echocardiographic image quality. Significant reduction in the V‐wave was observed in patients with (38±15 vs 24±10 mm Hg; P<0.001) and without (38±13 vs 30±9 mm Hg; P<0.001) a flail leaflet (A). Patients with a flail leaflet had a significantly larger reduction in the left atrial V‐wave compared with those without a flail leaflet (P=0.007). Significant reduction was also seen in all groups when stratified by number and location of regurgitant jets (B) and 3D echocardiographic image quality (C). Similar mean reduction in V‐wave was seen in patients with a single vs multiple jets (P=0.56) and fair vs high‐quality 3D echocardiographic image quality (P=0.31).
Multivariable predictors of optimal LAP reduction are shown in Table 3. Independent predictors of optimal LAP reduction include the presence of a flail leaflet (3.49; 95% confidence interval [CI], 1.40–9.15; P=0.007), 3D echocardiographic image quality graded as good or excellent (4.72; 95% CI, 1.16–32.2; P=0.03), and the presence of a single regurgitant jet or multiple jets that originate from a single scallop (3.56; 95% CI, 1.24–11.98; P=0.02).
Table 3.
Multivariable Predictors of Adequate LAP Reduction and Increased MnG Postprocedure
| Optimal LAP Reduction | MnG >5 mm Hg Postprocedure | ||||
|---|---|---|---|---|---|
| Parameter | Adjusted Risk Ratio (95% CI) | P Value | Parameter | Adjusted Risk Ratio (95% CI) | P Value |
| Flail scallop | 3.49 (1.40–9.15) | 0.007 | Preprocedural MnGa | 2.34 (1.27–5.07) | 0.005 |
| Single jet or multiple jets originating from a single scallop | 3.56 (1.24–11.98) | 0.02 | MAC | 12.21 (2.24–109.76) | 0.003 |
| Good or excellent 3D image quality | 4.72 (1.16–32.16) | 0.03 | >1 Clip deployed | 7.69 (1.62–48.6) | 0.009 |
3D indicates 3 dimensional; CI, confidence interval; LAP, left atrial pressure; MAC, mitral annular calcification; and MnG, mean diastolic gradient.
Per 1–mm Hg increase.
A composite score was created by assigning points to the previously described parameters in proportion to their adjusted risk ratio; 2 points were assigned for a flail leaflet, 2 points were assigned for a single regurgitant jet, and 3 points were assigned for good or excellent 3D image quality. Of 112 patients, a score of 0 was present in 7 (6%), a score of 2 was present in 10 (9%), a score of 3 was present in 19 (17%), a score of 4 was present in 6 (5%), a score of 5 was present in 43 (38%), and a score of 7 was present in 27 (24%). Patients with a higher score were more likely to achieve optimal LAP reduction postprocedure (Figure 2).
Figure 2.

Optimal left atrial pressure reduction according to imaging–anatomic composite score. Echocardiographic imaging–anatomic composite score was associated with the likelihood of successful left atrial pressure reduction postprocedure (P=0.0001). A score of 2 points was assigned for a flail scallop, a score of 2 points was assigned for a single regurgitant jet, and a score of 3 points was assigned for optimal 3‐dimensional echocardiographic image quality in proportion to their adjusted risk ratios. Optimal left atrial pressure reduction was not observed in patients with a score of 0 to 2 (n=17). By contrast, 56% of patients with all 3 favorable characteristics achieved optimal left atrial pressure reduction (score >5, n=27). Patients with a score of 3 to 5 had intermediate likelihood of optimal left atrial pressure reduction.
Predictors of Increased Mean Diastolic Gradient Postprocedure
Overall, 19 of 112 patients (17%) had a mitral valve mean diastolic gradient >5 mm Hg postprocedure. Pathoanatomic correlates of increased mean diastolic gradient are shown in Table 2. Patients with an increased mean gradient postprocedure had slightly higher preprocedural mean diastolic gradient (3±1 versus 2±1 mm Hg; P=0.01) and were more likely to have multiple regurgitant jets (68% versus 41%; P=0.03), >1 jet involving at least 2 scallops (58% versus 30%; P=0.02), mitral annular calcification (79% versus 37%; P=0.0009), and multiple clips implanted (68% versus 34%; P=0.006). Mean heart rate postprocedure did not differ in those with versus without increased diastolic gradient (77±14 versus 72±12 beats per minute; P=0.22). Independent predictors of increased mean diastolic gradient postprocedure included increased preprocedural mean gradient (P=0.005), mitral annular calcification (P=0.003; Figure 3), and the use of multiple clips (P=0.009; Table 3).
Figure 3.

Postprocedure mean diastolic gradient in patients with and without mitral annular calcification (MAC). Patients with MAC had increased mean diastolic gradient postprocedure compared with those without MAC (5±2 vs 4±1 mm Hg; P=0.003).
A composite score was created by assigning points to the previously described parameters in proportion to their adjusted risk ratio; 1 point was assigned for a preprocedure mean diastolic gradient >3 mm Hg, 3 points were assigned for implantation of >1 clip, and 5 points were assigned for mitral annular calcification. A score of 0 to 1 was present in 36 of 112 patients (32%), 3 to 5 in 49 of 112 patients (44%), and >5 in 24 of 112 patients (21%). Patients with a higher score were more likely to develop a mean diastolic gradient >5 mm Hg postprocedure (Figure 4).
Figure 4.
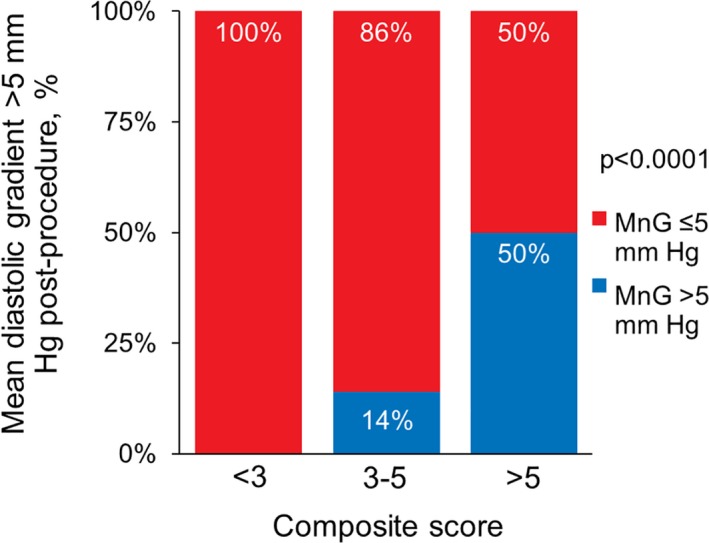
Mean gradient >5 mm Hg postprocedure, according to composite risk score. One point was assigned for a preprocedure mean gradient of >3 mm Hg, 3 points were assigned for >1 clip implanted, and 5 points were assigned for mitral annular calcification in proportion to the adjusted risk ratios. No patients with a score of <3 (n=36) developed a postprocedure mean diastolic gradient (MnG) of >5 mm Hg, but elevated postprocedure mean gradient was observed in 50% of patients with a score of >5 (n=24) (P<0.0001).
Patients with a postprocedural mean gradient >5 mm Hg had a similar percentage reduction in mean LAP (12±22% versus 11±25%; P=0.91) and a similar proportion with a mean LAP reduction >25% (16% versus 22%; P=0.52). However, preprocedural mean LAP (25±6 versus 21±5 mm Hg; P=0.005) and postprocedural mean LAP (22±5 versus 18±5 mm Hg; P=0.005) were higher in those with versus without a mean diastolic gradient >5 mm Hg postprocedure.
Interobserver Variability
Interobserver variability was performed on 20 patient echocardiograms. Excellent agreement was observed for identification of a flail scallop (κ coefficient, 1.00; 95% CI, 1.00–1.00), and moderate agreement was observed for A2‐P2 jet location (κ coefficient, 0.53; 95% CI, 0.14–0.92), identification of multiple jets involving >1 scallop (κ coefficient, 0.53; 95% CI, 0.14–0.92), and adequate (good‐to‐excellent) 3D image quality (κ coefficient, 0.44; 95% CI, 0.00–1.00).
Discussion
Identification of individuals with MR who will derive optimal outcomes with TMVR remains challenging. The present study is one of few to evaluate specific echocardiographic anatomic predictors of success in patients undergoing TMVR and the first to use directly measured LAP as a marker of optimal result. Specific echocardiographic predictors of optimal LAP reduction included the presence of flail leaflet, a single regurgitant jet or multiple regurgitant jets limited to a single scallop, and better 3D echocardiographic image quality. These data serve to advance the scientific approach to patient selection for TMVR therapy.
Reduction in the degree of MR and resultant LAP reduction are primary goals of TMVR. We have recently shown that larger left atrial V‐wave pressure reduction is associated with greater improvements in 6‐minute walk distance at 30 days after TMVR.9 Previous studies evaluating anatomic predictors of short‐term procedural success have been small, and many consisted of predominantly secondary (functional) MR.14, 15 A recent study using Transcatheter Valve Therapies Registry data of the early US experience with TMVR showed that left ventricular end‐diastolic dimension, A2‐P2 clip location, MR severity, and case volume were predictive of procedural success.16 One limitation of this large cross‐sectional cohort is that quantitative measurements of mitral valve anatomical features were not available for evaluation.
In this study of patients undergoing TMVR, the presence of a flail leaflet and a single jet of regurgitation or multiple jets isolated to a single scallop were independent predictors of optimal LAP reduction. Patients with a flail leaflet tended to have more severe MR, the origin of which was typically confined to a relatively small region of the mitral leaflets, making these lesions amenable to edge‐to‐edge repair (Figure 5; Videos S1 and S2). Similarly, patients with a single jet or multiple jets confined to a single scallop were more likely to have optimal reduction in LAP. Conversely, when multiple jets are present involving >1 scallop, it can be difficult to address each jet of MR without causing some degree of mitral stenosis (Figure 6; Videos S3 and S4). In some cases, the degree of mitral stenosis caused by an additional device may be prohibitive, prompting the treating physicians to accept some degree of residual MR and, thus, less reduction in LAP.
Figure 5.

Optimal left atrial pressure reduction in a patient with flail leaflet. Two‐dimensional color Doppler (A; Video S1) and 3‐dimensional (3D) (B; Video S2) echocardiographic images demonstrate a patient with a P2 flail scallop (*) and resultant severe mitral regurgitation. Only a single jet of regurgitation is present, and 3D imaging is high quality. Optimal left atrial pressure reduction was achieved (C) with a reduction in the V‐wave from 46 to 18 mm Hg (61% reduction) after deployment of 1 MitraClip. Echocardiographic imaging showed mild residual regurgitation.
Figure 6.

Left atrial pressure reduction in a patient with multiple regurgitant jets. Two‐dimensional color Doppler (A; Video S3) and 3‐dimensional color Doppler (B; Video S4) images demonstrate a patient with 3 regurgitant jets involving A1‐P1, A2‐P2, and A3‐P3. After implantation of 2 MitraClips, the V‐wave reduced from 34 to 26 mm Hg (24% reduction) and echocardiography showed moderate residual regurgitation. Mean diastolic gradient was 6 mm Hg, which precluded additional MitraClip implantation.
High‐quality 3D echocardiographic image quality was also independently associated with optimal reduction in LAP. This highlights the importance of having a precise understanding of the underlying mitral valve pathologic features and the location and severity of regurgitant jets. Precise and careful adjustment of the clip trajectory and clip arm orientation are critical steps in this procedure, and without adequate 3D echocardiographic image quality, the likelihood of success appears to be diminished. Echocardiographic grading of regurgitation after MitraClip also remains challenging. The presence of multiple jets or eccentric jets can lead to underestimation of the severity by 2‐dimensional images alone. By 3D echocardiography, the number, location, and extent of jets may be better appreciated. In the future, quantitative 3D echocardiography may prove more accurate in assessing the severity of regurgitation after clip and could be integrated with direct measurement of LAP to better estimate procedural success.
In the present study, 17% of patients had a mean diastolic gradient >5 mm Hg postprocedure. Independent predictors of an increased mean gradient postprocedure included an increased preprocedural mean gradient, mitral annular calcification, and implantation of multiple clips. Multiple MitraClip devices were implanted in 40% of our cohort. In many cases, optimal reduction of MR requires multiple devices and frequently there is a tradeoff between optimal reduction of MR (with an additional device) and development of mitral stenosis (attributable to mitral annular calcification and/or multiple devices). Taken together, our data suggest that optimal results are less likely in patients with even borderline elevated mean gradient preprocedure, mitral annular calcification, and multiple jets of MR that are likely to require multiple clips. In these cases, optimal MR reduction may be limited by resultant mitral stenosis after clip implantation. The mechanism by which mitral annular calcification leads to mitral stenosis in this cohort is not entirely clear. It may be attributable to progressive narrowing of the mitral annulus from bulky calcification or, alternatively, even a small degree of calcification that extends into the posterior mitral leaflet could result in restricted leaflet motion and stenosis. Further work is needed to clarify whether there is a threshold or location of mitral annular calcification that makes an individual more at risk of stenosis postprocedure.
We also noted an association between smaller left atrial volume index and higher residual mean gradient postprocedure, which may be related to smaller mitral annular size. Despite the increased mean diastolic gradient postprocedure, these patients derived a similar percentage reduction in mean LAP. However, patients with elevated mean gradients postprocedure had increased absolute mean LAP preprocedure and postprocedure. Thus, although it appears these patients derive similar hemodynamic benefit, there is likely an element of mitral stenosis in many of these patients that persists postprocedure. The clinical significance of this finding warrants further exploration.
LAP after TMVR is an important determinant of functional status.9 Although MR reduction likely plays an important role in LAP (V‐wave) changes, it is not the sole determinant. Additional contributions come from systemic preload, systemic afterload, relative mitral stenosis, left atrial compliance, and the iatrogenic atrioseptal defect created at the TMVR procedure. Although we would not expect major changes in systemic preload or afterload pre‐ versus post‐TMVR, it is likely that left atrial compliance also plays a role in the observed changes in LAP. Left atrial noncompliance has been demonstrated to be an important cause of elevated LAP in a variety of pathologic states.17, 18, 19, 20 Further work is needed to determine the significance of left atrial compliance as a contributor to LAP and subsequent outcome in TMVR.
Limitations
This study involved detailed echocardiographic review for anatomic mitral valve characteristics in a consecutive series of patients undergoing TMVR who underwent continuous LAP recording throughout the MitraClip procedure. The study is limited by a relatively small sample size, a single‐center design, and a study population that was selected to undergo TMVR on the basis of existing criteria. A larger cohort of patients could improve statistical power and reveal additional anatomic predictors of procedural success. Our cohort was also limited by the lack of an accurate estimate of mitral valve area because mitral valve planimetry was not possible retrospectively in most patients. We believe that pressure half‐time is not likely an accurate estimate of mitral valve area in this cohort with varying degrees of diastolic dysfunction, frequent arrhythmia, and frequent aortic valve disease.
Right‐sided heart catheterization data were not available in most patients, precluding analysis of changes in cardiac output and right‐sided pressures. However, LAP data are readily available as part of the MitraClip procedure, whereas right‐sided heart catheterization is not routinely performed, thus making the present study highly applicable to routine clinical practice.
Although the magnitude of the V‐wave is proportional to the mitral regurgitant volume, it can potentially be influenced by left atrial size, left atrial compliance, preload, and afterload. Although factors such as fluid administration during the MitraClip procedure and systemic blood pressure changes can alter LAP, LAP changes during MitraClip have been shown to independently predict improvement in 6‐minute walk distance at 30‐day follow‐up.9 Furthermore, systemic arterial pressure was not significantly different between groups preprocedure and postprocedure, minimizing the influence of this variable.
Conclusions
Mitral valve pathoanatomic echocardiographic characteristics, including flail leaflet and a single regurgitant jet, are predictive of optimal LAP reduction with TMVR. In addition, high‐quality 3D echocardiographic imaging was associated with optimal results. Increased mean diastolic gradient postprocedure was more likely with increased preprocedural mean gradient, mitral annular calcification, and implantation of multiple clips. These data expand current knowledge about patient selection for TMVR and deserve further study in larger cohorts.
Disclosures
None.
Supporting information
Video S1. 2D echocardiography with color Doppler imaging of a flail P2 mitral leaflet. A mid‐esophageal long axis view of the mitral valve demonstrates a flail P2 scallop (left panel) with severe mitral regurgitation (right panel). A single, discrete jet of mitral regurgitation is present.
Video S2. 3D echocardiographic left atrial view of a flail P2 mitral scallop. A 3D echocardiographic view of the mitral valve from the left atrial perspective (“surgeon's view”) demonstrates very clearly a flail P2 scallop with ruptured chordae tendinea.
Video S3. 2D echocardiography with color Doppler imaging of three mitral regurgitant jets. A mid‐esophageal commissural view demonstrates 3 jets of mitral regurgitation spanning the width of the mitral valve from lateral to medial (A1‐P1, A2‐P2, and A3‐P3).
Video S4. 3D echocardiography with color Doppler demonstrating three jets of mitral regurgitation. 3D color Doppler imaging from the left atrial perspective (“surgeon's view”) demonstrates three jets of mitral regurgitation. The dominant jet appears to arise from the A1‐P1 scallops (seen laterally, on the left side of the image).
(J Am Heart Assoc. 2018;7:e007315 DOI: 10.1161/JAHA.117.007315.)29331957
References
- 1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez‐Sarano M. Burden of valvular heart diseases: a population‐based study. Lancet. 2006;368:1005–1011. [DOI] [PubMed] [Google Scholar]
- 2. Mirabel M, Iung B, Baron G, Messika‐Zeitoun D, Detaint D, Vanoverschelde JL, Butchart EG, Ravaud P, Vahanian A. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J. 2007;28:1358–1365. [DOI] [PubMed] [Google Scholar]
- 3. Feldman T, Kar S, Rinaldi M, Fail P, Hermiller J, Smalling R, Whitlow PL, Gray W, Low R, Herrmann HC, Lim S, Foster E, Glower D; EVEREST Investigators . Percutaneous mitral repair with the mitraclip system: safety and midterm durability in the initial EVEREST (Endovascular Valve Edge‐to‐Edge REpair Study) cohort. J Am Coll Cardiol. 2009;54:686–694. [DOI] [PubMed] [Google Scholar]
- 4. Mauri L, Foster E, Glower DD, Apruzzese P, Massaro JM, Herrmann HC, Hermiller J, Gray W, Wang A, Pedersen WR, Bajwa T, Lasala J, Low R, Grayburn P, Feldman T; EVEREST II Investigators . 4‐Year results of a randomized controlled trial of percutaneous repair versus surgery for mitral regurgitation. J Am Coll Cardiol. 2013;62:317–328. [DOI] [PubMed] [Google Scholar]
- 5. Conradi L, Treede H, Rudolph V, Graumuller P, Lubos E, Baldus S, Blankenberg S, Reichenspurner H. Surgical or percutaneous mitral valve repair for secondary mitral regurgitation: comparison of patient characteristics and clinical outcomes. Eur J Cardiothorac Surg. 2013;44:490–496; discussion 496 [DOI] [PubMed] [Google Scholar]
- 6. Paranskaya L, D'Ancona G, Bozdag‐Turan I, Akin I, Kische S, Turan GR, Rehders T, Schneider H, Westphal B, Birkemeyer R, Nienaber CA, Ince H. Percutaneous vs surgical repair of mitral valve regurgitation: single institution early and midterm outcomes. Can J Cardiol. 2013;29:452–459. [DOI] [PubMed] [Google Scholar]
- 7. Taramasso M, Denti P, Buzzatti N, De Bonis M, La Canna G, Colombo A, Alfieri O, Maisano F. Mitraclip therapy and surgical mitral repair in patients with moderate to severe left ventricular failure causing functional mitral regurgitation: a single‐centre experience. Eur J Cardiothorac Surg. 2012;42:920–926. [DOI] [PubMed] [Google Scholar]
- 8. Mauri L, Garg P, Massaro JM, Foster E, Glower D, Mehoudar P, Powell F, Komtebedde J, McDermott E, Feldman T. The EVEREST II Trial: design and rationale for a randomized study of the evalve mitraclip system compared with mitral valve surgery for mitral regurgitation. Am Heart J. 2010;160:23–29. [DOI] [PubMed] [Google Scholar]
- 9. Maor E, Raphael CE, Panaich SS, Reeder GS, Nishimura RA, Nkomo VT, Rihal CS, Eleid MF. Acute changes in left atrial pressure after mitraclip are associated with improvement in 6‐minute walk distance. Circ Cardiovasc Interv.2017;10:e004856. [DOI] [PubMed] [Google Scholar]
- 10. Eleid MF, Reeder GS, Malouf JF, Lennon RJ, Pislaru SV, Nkomo VT, Rihal CS. The learning curve for transcatheter mitral valve repair with mitraclip. J Interv Cardiol. 2016;29:539–545. [DOI] [PubMed] [Google Scholar]
- 11. Eleid MF, Sanon S, Reeder GS, Suri RM, Rihal CS. Continuous left atrial pressure monitoring during mitraclip: assessing the immediate hemodynamic response. JACC Cardiovasc Interv. 2015;8:e117–e119. [DOI] [PubMed] [Google Scholar]
- 12. Carpentier AF, Lessana A, Relland JY, Belli E, Mihaileanu S, Berrebi AJ, Palsky E, Loulmet DF. The “physio‐ring”: an advanced concept in mitral valve annuloplasty. Ann Thorac Surg. 1995;60:1177–1185; discussion 1185–1186. [DOI] [PubMed] [Google Scholar]
- 13. Zoghbi WA, Enriquez‐Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ; American Society of Echocardiography . Recommendations for evaluation of the severity of native valvular regurgitation with two‐dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. [DOI] [PubMed] [Google Scholar]
- 14. Lubos E, Schluter M, Vettorazzi E, Goldmann B, Lubs D, Schirmer J, Treede H, Reichenspurner H, Blankenberg S, Baldus S, Rudolph V. Mitraclip therapy in surgical high‐risk patients: identification of echocardiographic variables affecting acute procedural outcome. JACC Cardiovasc Interv. 2014;7:394–402. [DOI] [PubMed] [Google Scholar]
- 15. Taramasso M, Denti P, Latib A, Guidotti A, Buzzatti N, Pozzoli A, Di Giannuario G, La Canna G, Colombo A, Alfieri O, Maisano F. Clinical and anatomical predictors of mitraclip therapy failure for functional mitral regurgitation: single central clip strategy in asymmetric tethering. Int J Cardiol. 2015;186:286–288. [DOI] [PubMed] [Google Scholar]
- 16. Sorajja P, Mack M, Vemulapalli S, Holmes DR Jr, Stebbins A, Kar S, Lim DS, Thourani V, McCarthy P, Kapadia S, Grayburn P, Pedersen WA, Ailawadi G. Initial experience with commercial transcatheter mitral valve repair in the United States. J Am Coll Cardiol. 2016;67:1129–1140. [DOI] [PubMed] [Google Scholar]
- 17. Kihara Y, Sasayama S, Miyazaki S, Onodera T, Susawa T, Nakamura Y, Fujiwara H, Kawai C. Role of the left atrium in adaptation of the heart to chronic mitral regurgitation in conscious dogs. Circ Res. 1988;62:543–553. [DOI] [PubMed] [Google Scholar]
- 18. Ko YG, Ha JW, Chung N, Shim WH, Kang SM, Rim SJ, Jang Y, Cho SY, Kim SS. Effects of left atrial compliance on left atrial pressure in pure mitral stenosis. Catheter Cardiovasc Interv. 2001;52:328–333. [DOI] [PubMed] [Google Scholar]
- 19. Heywood TJ, Seethala S, Khan T, Johnson A, Smith M, Rubenson D, Reynolds E. Left atrial diastolic dysfunction and pulmonary venous hypertension in atrial fibrillation: clinical, hemodynamic and echocardiographic characteristics. J Atr Fibrillation. 2014;7:1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. 2015;8:295–303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. 2D echocardiography with color Doppler imaging of a flail P2 mitral leaflet. A mid‐esophageal long axis view of the mitral valve demonstrates a flail P2 scallop (left panel) with severe mitral regurgitation (right panel). A single, discrete jet of mitral regurgitation is present.
Video S2. 3D echocardiographic left atrial view of a flail P2 mitral scallop. A 3D echocardiographic view of the mitral valve from the left atrial perspective (“surgeon's view”) demonstrates very clearly a flail P2 scallop with ruptured chordae tendinea.
Video S3. 2D echocardiography with color Doppler imaging of three mitral regurgitant jets. A mid‐esophageal commissural view demonstrates 3 jets of mitral regurgitation spanning the width of the mitral valve from lateral to medial (A1‐P1, A2‐P2, and A3‐P3).
Video S4. 3D echocardiography with color Doppler demonstrating three jets of mitral regurgitation. 3D color Doppler imaging from the left atrial perspective (“surgeon's view”) demonstrates three jets of mitral regurgitation. The dominant jet appears to arise from the A1‐P1 scallops (seen laterally, on the left side of the image).


