Calcific aortic valve disease (CAVD) is the most common form of valvular heart disease worldwide and is associated with increased mortality across the spectrum of severity of disease.1 The 1‐year mortality rates among individuals with untreated severe symptomatic aortic stenosis nears 50%.2 Because of rapid population aging, global CAVD prevalence is expected to double in the next 50 years.3
More important, there are no available therapies to effectively prevent the onset or slow the progression of CAVD. Although substantial progress has been made in the identification of novel risk factors for development of the disease,4 in the use of multi‐imaging modalities for better diagnosis and staging,5 and in the development of less invasive treatment strategies for those with severe aortic stenosis,1 disease prevention may be most optimally achieved through health behavior change.
In this issue of Journal of the American Heart Association (JAHA),6 Sengeløv and colleagues present results from an analysis of 6034 participants in the ARIC (Atherosclerosis Risk in Communities) Study. They evaluate the association between prevalent CAVD and health behaviors plus modifiable risk factors assessed through the use of a combined metric of “ideal cardiovascular health, ” the American Heart Association cardiovascular health score (CVHS).7
Greater attainment of ideal cardiovascular health was associated with a lower prevalence of CAVD, with a 60% reduction in the prevalent odds of aortic valve calcification among participants with highest attained CVHS score (CVHS >80%) compared with those in the lowest CVHS group (CVHS <50%). Notably, trajectories in attainment of CVHS score suggest a continued benefit of better health behaviors and risk factor control past the age of 50 years. This implies that the benefit of a healthy lifestyle extends to older individuals.
Although these results are consistent with other observational studies evaluating the association between modifiable cardiovascular risk factors and CAVD,8 causality cannot be inferred. Because the time of onset of CAVD in relation to the exposure is unknown, the temporal relationship between health behaviors and modifiable risk factors with the onset of CAVD cannot be established. In addition, the study population represented only 40% of the original ARIC Study cohort. These select participants may have a different risk factor profile and association with CAVD than those who were no longer being observed. In addition, most had mild CAVD, with few individuals having moderate‐to‐severe aortic stenosis; this is an important consideration given that risk factors for aortic sclerosis may be different than those associated with severe valve disease.9
In addition, the definition of CAVD in the current study is not ideal. In the ARIC Study, echocardiographic diagnosis of CAVD was classified by the degree of hemodynamic obstruction, with aortic sclerosis defined as a peak velocity between 1.5 and 2.0 m/s. We think aortic sclerosis is better defined by anatomical features, specifically thickening and calcification of the valve leaflets, with Doppler velocities used only to ensure that valve obstruction is not present.
The inherent limitation of drawing casual inferences from observational studies of modifiable cardiovascular risk factors in CAVD is demonstrated by the results of 3 randomized clinical trials that showed no significant effect of statins and aggressive cholesterol reduction in slowing the progression of aortic stenosis,10, 11, 12 despite observational studies showing strong associations between cholesterol levels and progression of valve disease.13 These results highlight the substantial knowledge gap that exists in the identification of proximal determinants of the phenotypic expression of CAVD over other chronic systemic inflammatory conditions, despite common upstream determinants, and in the risk factors accounting for the progression of the disease (Figure). Filling this gap will require innovative studies integrating novel genomic and phenotypic information, systems biology, and high‐sensitivity imaging modalities that would make the discovery of an effective medical therapy for calcific CAVD more likely.
Figure 1.
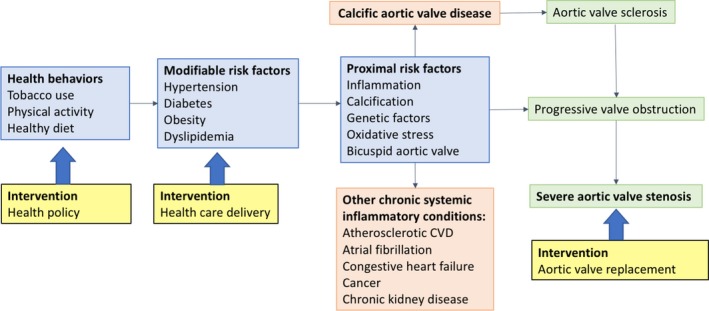
Conceptual framework for prevention of calcific aortic valve disease (CAVD). The current approach to treatment of CAVD is valve replacement for end‐stage disease (severe aortic stenosis). Although there is ongoing basic and clinical research into targeted therapies of proximal risk factors that might prevent disease progression, none have been shown to be effective to date. In addition to these ongoing research efforts, more attention should be focused on public policies to increase health behaviors at the population level and to improve healthcare delivery of known primary cardiovascular disease (CVD) prevention strategies.
Although more and better research is needed to elucidate proximal risk factors and pathways leading to aortic valve disease, the knowledge that healthy behaviors are beneficial in preventing virtually any chronic nontransmissible systemic inflammatory disease is well documented. For example, health behaviors in the form of healthy eating, regular physical activity, and abstinence from smoking are the main upstream determinants of atherosclerotic cardiovascular disease.14 Furthermore, low rates of atherosclerotic cardiovascular disease have been demonstrated in individuals adhering to a healthy lifestyle, even when having a high genetic predisposition for the disease.15 Given their shared risk factors, it is plausible that modification of health behaviors would concurrently decrease the incidence of both atherosclerotic cardiovascular disease and CAVD.
Yet, effectively modifying health behaviors at the population level remains a public health conundrum. Herein, the problem lies in introducing and evaluating better policy solutions to effectively promote healthy behaviors and in the control of modifiable risk factors. Although significant success has been achieved in lowering tobacco use rates and in the control of blood pressure and cholesterol levels with medications, much less progress has been achieved in promoting healthy eating, encouraging regular physical activity, and controlling obesity.16 With current trends, it is projected that half of all US adults will be obese by 2030.17
The lack of progress in promoting and achieving a healthy lifestyle is, in part, attributable to ongoing uncertainty of the effectiveness of many proposed and implemented policy solutions aimed at health behavior modification. For example, the “soda tax” has gained international notoriety for its potential to decrease sugar consumption by deterring purchase of sugar‐sweetened beverages.18 However, evaluations to date have focused on policy outputs (eg, changes in quantity of sugar‐sweetened beverage purchased) but have stopped short of assessing impacts on health outcomes.19 To be considered effective, evidenced‐based public policy solutions aimed at changing health behaviors need to be thoroughly and systematically evaluated to determine if and how they achieve protective health benefits.20 Rigorous population‐level trials are necessary to identify how such policy solutions can be optimized to change behavior and prevent disease.
Although an effective therapy to prevent the onset and slow the progression of calcific CAVD is far from becoming a reality, the best and most cost‐effective intervention for the prevention of this and other chronic diseases may be the promotion of healthy behaviors. We must shift our focus and investment to the investigation of effective policy solutions to modify these behaviors at a population level.
Disclosures
None.
J Am Heart Assoc. 2018;7:e008385e008385. DOI: 10.1161/JAHA.117.008385.29431108
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
References
- 1. Lindman BR, Clavel MA, Mathieu P, Lung PL, Otto CM, Pibarot P. Calcific aortic stenosis. Nat Rev. 2016;2:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leon MB, Smith CR, Mack M, Miller G, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Hermmann HC, Douglas PD, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S. Transcatheter aortic‐valve implantation for aortic stenosis in patient who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. [DOI] [PubMed] [Google Scholar]
- 3. Lung B, Vahanian A. Degenerative calcific aortic stenosis: a natural history. Heart. 2012;98:7–13. [DOI] [PubMed] [Google Scholar]
- 4. Thanassoulis G, Campbell CY, Owens DS, Smith JG, Smith AV, Peloso GM, Kerr KF, Pechlivanis S, Budoff MJ, Harris TB, Malhotra R, O'Brien KD, Kampstrup PR, Nordestgaard BG, Tybjaerg‐Hansen A, Allison MA, Aspelund T, Criqui MH, Heckbert SR, Hwang SJ, Liu Y, Sjogren M, van der Pals J, Kälsch H, Mühleisen TW, Nöthen MM, Cupples LA, Caslake M, Di Angelantonio E, Danesh J, Rotter JI, Sigurdsson S, Wong Q, Erbel R, Kathiresan S, Mellander O, Gudnason V, O'donnell CJ, Post W. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dulgheru R, Pibarot P, Sengupta P. Multimodality imaging strategies for the assessment of aortic stenosis. Circ Cardiovasc Imaging. 2016;9:e004352. [DOI] [PubMed] [Google Scholar]
- 6. Sengeløv M, Cheng S, Biering‐Sørensen T, Matsushita K, Konety S, Solomon S, Folsom A, Shah A. Ideal cardiovascular health and the prevalence and severity of aortic stenosis in the elderly. J Am Heart Assoc. 2018;7:e007234 DOI: 10.1161/JAHA.117.007234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lloyd‐Jones DM, Hong YH, Labarthe D. Defining and setting national goals for cardiovascular health promotion and disease reduction. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 8. Katz R, Wong ND, Kronmal R, Takasu J, Shavelle DM, Probstfield JL, Bertoni AG, Budoff MJ, O'Brien KD. Features of the metaoblic syndrome and diabetes mellitus as predictors of aortic valve calcification in the Multi‐Ethnic Study of Atherosclerosis. Circulation. 2006;113:2113–2119. [DOI] [PubMed] [Google Scholar]
- 9. Rajamannan NM, Evans F, Aikawa E, Grande‐Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O'Brien KD, Schoen FJ, Towler DA, Yoganathan AP, Otto C. Calcific aortic valve disease: not simply a degenerative process. Circulation. 2011;124:1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, Boon NA. A randomized trial of intensive lipid‐lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352:2389–2397. [DOI] [PubMed] [Google Scholar]
- 11. Rossebo A, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, Gerdts E, Gohlke‐Bärwolf C, Holme I, Kesäniemi YA, Malbecq W, Nienaber CA, Ray S, Skjærpe T, Wachtell K, Willenheimer R. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. [DOI] [PubMed] [Google Scholar]
- 12. Chan KL, Teo K, Dumesnil JG, Ni A, Tam J. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis. Circulation. 2010;121:306–314. [DOI] [PubMed] [Google Scholar]
- 13. Aronow WS, Ahn C, Kronzon I, Goldman ME. Association of coronary risk factors and use of statins with progression of mild valvular aortic stenosis in older persons. Am J Cardiol. 2001;88:693–695. [DOI] [PubMed] [Google Scholar]
- 14. Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2010;343:16–22. [DOI] [PubMed] [Google Scholar]
- 15. Khera AV, Emdin CA, Drake I, Natarajan P, Bick AG, Cook NR, Chasman DI, Baber U, Mehran R, Rader DJ, Fuster V, Boerwinkle E, Melander O, Orho‐Melander M, Ridker PM, Kathiresan S. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med. 2016;375:2349–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. US Department of Health and Human Services . Healthy People 2020. http://www.healthypeople.gov/2020/default.aspx. Accessed January 25, 2018.
- 17. Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378:815–825. [DOI] [PubMed] [Google Scholar]
- 18. Sugar: a taxing problem? Editorial. Lancet Oncol. 2015;16:1569. [DOI] [PubMed] [Google Scholar]
- 19. Colchero MA, Popkin BM, Rivera JA, Ng SW. Beverage purchases from stores in Mexico under the excise tax on sugar sweetened beverages: observational study. BMJ. 2016;352:h6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baicker K, Chandra A. Evidence‐based health policy. N Engl J Med. 2017;377:2413–2415. [DOI] [PubMed] [Google Scholar]


