Abstract
This study was conducted to test the effects of dietary supplementation of feed grade L-Met on growth performance and redox status of turkey poults compared with the use of conventional DL-Met. Three hundred and eighty five newly hatched turkey poults were weighed and allotted to 5 treatments in a completely randomized design and the birds were fed dietary treatments for 28 d, including a basal diet (BD), the BD + 0.17 or 0.33% DL-Met or L-Met (representing 60, 75, and 90% of the requirement by National Research Council (NRC) for S containing AA, respectively). Increasing Met supplementation from 0 to 0.33% increased (P < 0.05) weight gain (690 to 746 g) and feed intake (1,123 to 1,248 g) of turkey poults. Supplementing L-Met tended (P = 0.053) to reduce feed to gain ratio (1.70 to 1.63) compared with DL-Met. The relative bioavailability of L-Met to DL-Met was 160% based on a multilinear regression analysis of weight gain. Supplementing Met regardless of its sources decreased (P < 0.05) malondialdehyde (3.29 to 2.47 nmol/mg protein) in duodenal mucosa compared with birds in the BD. Supplementing L-Met tended (P = 0.094) to decrease malondialdehyde (1.27 to 1.16 nmol/mg protein) and increase glutathione (3.21 to 3.45 nmol/mg protein) in the liver compared with DL-Met. Total antioxidant capacity, protein carbonyl, and morphology of duodenum and jejunum were not affected by Met sources. In conclusion, dietary supplementation of 0.33% Met to a diet with S containing AA meeting 60% of the NRC requirement enhanced weight gain, feed intake, and redox status by reducing oxidative stress in the gut and liver of turkey poults during the first 28 d of age. Use of L-Met tended to enhance feed efficiency and was more effective in reducing oxidative stress and increasing glutathione in the liver compared with the use of DL-Met. The use of L-Met as a source of Met replacing DL-Met seems to be beneficial to turkey poults during the first 28 d of age.
Keywords: growth performance, methionine, oxidative stress, turkey poult
INTRODUCTION
Indispensable amino acid (AA) are essential for normal growth and health for animals including turkeys (Baker, 2009). Typical poultry feed used in the United States is based on corn and soybean meal are often deficient in indispensable AA such as Met and Lys for the optimal growth (Fernandez et al., 1994; Pack et al., 2003). In commercial poultry production, Met, Lys, Thr, and Trp are typically supplemented to corn and soybean meal based diets.
Animal cells can utilize only L-AA for protein synthesis which are naturally existing in animal and plant tissues. Whereas, D-AA, if ingested, need to be converted to L-AA to be biologically available. The conversion of D-AA to L-AA is shown to occur in the liver and kidney in a chicken (Dibner and Knight, 1984). DL-methionine has widely been used as a Met source which is chemically synthesized and thus including both D and L isomers at 1:1 ratio (Baker and Boebel, 1980). In chicken, D-Met is shown to be effectively converted to L-Met in the liver (Dibner and Knight, 1984; Baker, 2006; Thwaites and Anderson, 2007) and utilized for protein synthesis and other metabolism pathways whereas it has not been shown in turkey.
The gut is the main consumer of dietary AA because 40 to 50% of absorbed AA are utilized in the gut (Stoll et al., 1998) and only the remaining AA enter the circulation to be used in the body. Only L-Met can be utilized by the tissues, whereas D-Met cannot be utilized by the tissues until it is converted to L-Met in the liver (Dibner and Knight, 1984). Therefore, only L-Met can be metabolized to glutathione and taurine to function as antioxidants in the gut and the liver (Finkelstein, 1990), whereas D-Met cannot help the redox status in those tissues until being converted to L-Met (Shen et al., 2014, 2015). If Met can be supplemented entirely as L-Met, it may enhance the utilization of Met in the tissues to help the redox status compared with the use of DL-Met (Luo and Levine, 2009; Shen et al., 2014, 2015).
It is hypothesized that the use of L-Met replacing DL-Met can enhance the redox status in the gut and hepatic tissue and thus enhance gut health and growth performance of turkeys. The objective of this study was to determine growth performance and redox status in the gut and hepatic tissues of turkey poults receiving diets with different levels of Met from 2 sources (L-Met and DL-Met).
MATERIALS AND METHODS
The experimental protocol was approved by the Institutional Animal Care and Use Committee at North Carolina State University (Raleigh, NC).
Animal and Design
The experiment was conducted in the Prestage Department of Poultry Sciences, at NC State University (Raleigh, NC). Three hundred eighty five newly hatched Nicholas turkey poults (Prestage Farms, Clinton, NC) were weighed and randomly allotted to 5 dietary treatments in a randomized complete block design on the day of hatching. Dietary treatments included a basal diet (BD), the BD + 0.17% DL-Met or L-Met, the BD + 0.33% DL-Met or L-Met [representing 60, 75, and 90% of S containing AA (SCAA) requirement by National Research Council (NRC)]. In the beginning of the study, each treatment contained 7 cages with 11 turkey poults per cage. The cage was considered the experimental unit. Turkey poults were reared in 35 cages in a windowless room with controlled ventilation and temperature for 28 d. The room contained 4 sets of 12-cage (Alternative Design Manufacturing and Supply, Inc., Siloam Springs, AR). Each cage was 0.55 m in width by 0.66 m deep, and 0.36 m high equipped with a frontal feeder and 2 nipple drinkers. Turkey poults had ad libitum access to water and feed throughout the study. Feed additions were weighed and recorded. The turkey poults and feed were weighed on d 0, 7, 14, 21, and 28 for evaluation of growth performance. Dead turkey poults were removed and weighed daily to calculate mortality in weekly basis, and adjusted the growth performance data following Shen et al. (2015). Lighting program started with 23 h of light from 1 to 7 d, 22 h of light to 14 d, 20 h of light to 21 d and 18 h of light to 28 d. The temperature from hatching to 7 d was maintained at 32 to 34°C, reduced to 29°C until 14 d, reduced to 27°C until 21 d, and reduced to 25°C until 28 d.
All diets were prepared in a mash form. The basal diet was formulated to be deficient in SCAA according to the NRC (1994) requirement (Table 1). The methionine and SCAA in the BD were 0.32 and 0.63%, respectively meeting 60% of the NRC requirement, whereas other AA in the BD met the requirement suggested by NRC (1994). The dietary treatments supplemented with increasing levels of either DL-Met or L-Met brought Met content to 75 or 90% of the SCAA requirement. Supplemental feed grade L-Met was obtained from CJ CheilJedang Co. (Seoul, Korea), whereas DL-Met was commercially available and purchased locally by North Carolina State University Feed Mill (Raleigh, NC). Test diets were prepared by adding the different sources of Met to single common batch of the BD to minimize unintended variations. Analyzed levels of supplemental DL-Met and L-Met were 0.161 and 0.329% for 75% SCAA requirement or 0.159 and 0.327% for 90% SCAA requirement, respectively (Table 1).
Table 1.
Composition of the basal diet (%, as-fed basis).1
| Ingredient, % | |
|---|---|
| Corn | 40.68 |
| Soybean meal, dehulled | 48.32 |
| L-Lys HCl | 0.38 |
| L-Thr | 0.09 |
| Poultry fat | 4.81 |
| Ground limestone | 1.45 |
| Dicalcium phosphate, P 18.5% | 3.38 |
| Micro salt | 0.37 |
| Sodium Se premix | 0.05 |
| Choline chloride, 60% | 0.24 |
| Mineral premix2 | 0.13 |
| Vitamin premix3 | 0.10 |
| Total | 100.00 |
| Calculated nutrient composition | |
| DM, % | 89.7 |
| ME, Mcal/kg | 3.02 |
| CP, % | 26.7 |
| Lys, % | 1.84 |
| Thr, % | 1.11 |
| Met, % | 0.32 |
| Met + Cys, % | 0.63 |
| Ca, % | 1.40 |
| Available P, % | 0.75 |
| Total P, % | 1.02 |
1The basal diet contained 0.63% S containing AA meeting 60% of NRC requirement for a turkey poult from d 0 to 28 of age. The dietary treatments were supplemented with increasing levels (0.17 or 0.33%) of either DL-Met (analyzed levels: 0.161 and 0.329%) or L-Met (analyzed levels: 0.159 and 0.327%) to include S containing AA meeting 75 and 90% of NRC requirement, respectively.
2The trace mineral premix provided in milligrams per kilogram of complete diet: 4.0 mg of Mn as manganous oxide; 165 mg of Zn as zinc sulfate; 165 mg of Fe as ferrous sulfate; 16.5 mg of Cu as copper sulfate; 0.30 mg of I as ethylenediamine dihydroiodide.
3The vitamin premix provided per kilogram of diet: 6613.8 IU vitamin A as vitamin A acetate; 992.0 IU of vitamin D3; 19.8 IU vitamin E; 2.64 mg vitamin K as menadione sodiu, bisulfate; 0.03 mg of vitamin B12; 4.63 mg riboflavin; 18.52 mg of D-pantothenic acid as calcium panthonate; 24.96 mg of niacin; 0.07 mg biotin.
Sample Collection and Processing
On d 7 and 28, 1 bird representing the average weight of each cage was selected and euthanized from all treatments. After euthanasia, the gut and liver from BD, DL-Met (0.33%), and L-Met (0.33%) treatment were quickly dissected to determine redox status. The middle section of the duodenum was isolated and flushed gently with saline solution. Half of the isolated duodenum was fixed with 10% formaldehyde-phosphate buffer and kept for microscopic assessment of mucosal morphology (Shen et al., 2015). The other half was then opened for scraping the mucosa layer of the gut. The duodenal mucosa was scraped into 2.0 mL capacity microcentrifuge tube and frozen in liquid nitrogen. A part of the liver was also collected into 2.0 mL capacity microcentrifuge tube and snap frozen in liquid nitrogen. Mucosa and liver samples were then stored at −80°C until analyzed for concentrations of glutathione (GSH), total antioxidant capacity (TAC), protein carbonyl (PC), and malondialdehyde (MDA) as makers for redox status.
Glutathione
Glutathione was measured to determine antioxidant status. Duodenal mucosa (500 mg) and liver (500 mg) were weighed and suspended into 1.0 mL ice-cold phosphate-buffered saline (PBS) containing 5% metaphosphoric acid (Shen et al., 2014). Samples were homogenized using a glass pestle on ice. The homogenate was centrifuged at 15,000 × g at 4°C for 30 min. The supernatant was used to determine concentrations of GSH using an ELISA kit (STA-312; Cell Biolabs, San Diego, CA) and protein concentrations using a BCA protein assay kit (23,225; Thermo Scientific, Rockford, IL) (Shen et al., 2012). Concentrations of GSH were expressed as nmol/mg protein. The assay range for GSH was 0.0 to 0.5 μM with a sensitivity of < 8 nM.
Total Antioxidant Capacity
Total antioxidant capacity was measured to determine the capacity of cells to deal with reactive oxygen species (ROS) and free radicals. Duodenal mucosa (500 mg) and liver (500 mg) were weighed, suspended into 1.0 mL PBS, and homogenized using a Tissuemiser (Fisher Scientific, Pittsburgh, PA) on ice. The homogenate was centrifuged at 15,000 × g at 4°C for 30 min. The supernatant was used to determine concentrations of TAC using an ELISA kit (STA-360; Cell Biolabs) and protein concentrations using a commercial kit as mentioned in the GSH analysis. Concentrations of TAC were expressed as U/mg protein. Unit (U) means μM copper reducing equivalents per mg of protein. The assay range for TCA was 0 to 50 μM of uric acid with a sensitivity of <0.5 μM.
Malondialdehyde
Concentrations of MDA in duodenal mucosa and liver, as an index of lipid peroxidation, were analyzed using an ELISA kit (STA-330; Cell Biolabs) as described by Zhao et al. (2013). Duodenal mucosa (500 mg) and liver (500 mg) were weighed and suspended into 1.0 mL PBS containing 0.05% butylated hydroxytoluene. The homogenized was prepared as mentioned in the TAC analysis. The supernatant was used to determine concentrations of MDA and protein concentrations using a commercial kit as mentioned in the GSH analysis. Concentrations of MDA were expressed as nmol/mg protein. The assay range for MDA was 0 to 125 μM.
Protein Carbonyl
Protein carbonyl in mucosa of the duodenum and liver, as an index of oxidative stress were analyzed using an ELISA kit (STA 310; Cell Biolabs). Duodenal mucosa (500 mg) and liver (500 mg) were weighed, suspended into 1.0 mL PBS, and homogenized on ice (Zhao et al., 2013). The homogenate was prepared as mentioned in the TAC analysis. The supernatant was used to determine concentrations of PC and protein concentrations using a commercial kit as mentioned in the GSH. Concentrations of PC were expressed as nmol/mg protein. The assay range for PC was 0 to 7.5 nmol/mg protein.
Small Intestinal Morphometry
The segments of the duodenum and jejunum were embedded in paraffin, cut across the section to 5-mM-thick slides, and mounted on a polylysine-coated slide. Then, slides were stained (with hematoxylin and eosin) and examined under a Lumenera Infinity 3 digital camera (Lumenera Corporation, Ottawa, Canada) attached to an Olympus CX31 microscope (Olympus, Tokyo, Japan). Villus height (from the tip of the villi to the villous-crypt junction), villus width (width of the villus at one-half of the villus height), and crypt depth (from this junction to the base of the crypt) were determined (Shen et al., 2015). Lengths of 10 well oriented intact villi and their associated crypt were measured in each slide. The same person executed all the analysis of intestinal morphology.
Statistical Analysis
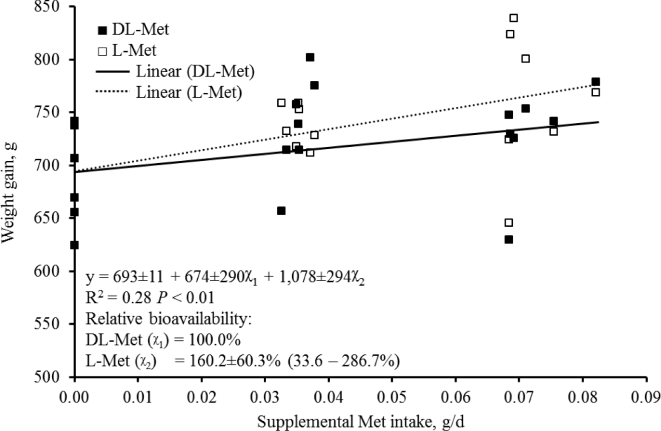
Data for growth performance (Table 2) were analyzed using Mixed Model (PROC MIXED) of SAS (SAS Inst. Inc., Cary, NC). The study was based on a randomized complete block design. The cage was considered the experimental unit. Room was a block factor. For growth performance, preplanned contrasts were used to evaluate the effects of Met source (Sc), supplemental levels of Met (Lv), and the interaction (Lv × Sc). For other physiological changes, statistical differences among treatments were determined by the PDIFF option of SAS. To evaluate the relative bioavailability (RBA) of L-Met to DL-Met for weight gain, multilinear regression equations were obtained by the PROC GLM of SAS (Littell et al., 1997; Kim and Easter, 2001; Ji et al., 2006). The following multilinear regression was applied:
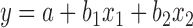 |
in which y = weight gain (WG), a = intercept (WG achieved with the BD), b1 = the slope of DL-Met line, b2= the slope of L-Met line, x1= intake of supplemental of DL-Met, and x2 = intake of supplemental of L-Met. The RBA values (%) of L-Met to DL-Met were given by the ratio of the slope coefficients, b2/b1, as described by Shen et al. (2015). Statistical differences were considered significant with P < 0.05, whereas 0.05 ≤ P < 0.10 was used as the criteria for tendency.
Table 2.
Growth performance of turkey poults fed diets with supplemental methionine from d 0 to 28 of age.1
| Supplemental | Supplemental | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| DL-Met, % | L-Met, % | P value | |||||||
| Item | BD2 | 0.17 | 0.33 | 0.17 | 0.33 | SEM | Lv3 | Sc4 | Lv × Sc |
| Initial BW, g | 59.8 | 59.4 | 60.3 | 59.4 | 59.4 | 0.4 | 0.59 | 0.44 | 0.57 |
| Weight gain, g | 690 | 737 | 730 | 738 | 762 | 19 | 0.012 | 0.46 | 0.60 |
| Feed intake, g | 1123 | 1230 | 1257 | 1212 | 1239 | 32 | 0.001 | 0.62 | 0.95 |
| Feed to gain ratio | 1.63 | 1.67 | 1.73 | 1.64 | 1.62 | 0.03 | 0.30 | 0.053 | 0.19 |
1Each mean represents 12 cages of 11 turkey poults per pen.
2BD = basal diet.
3Lv = supplemental levels (0, 0.17, and 0.33%) of Met.
4Sc = sources of Met (DL-Met and L-Met).
RESULTS
Growth Performance
Initial BW of birds did not differ among treatments (Table 2). Weight gain of birds during 28 d feeding period increased (P < 0.05) from 690 to 746 g (an average of 0.33% DL-Met and L-Met) as dietary Met supplementation regardless of Met source increased from 0 to 0.33% (Table 2). However, the source of Met did not affect the weight gain of birds. Feed intake (FI) of birds during 28 d feeding period increased (P < 0.05) from 1123 to 1248 g as Met supplementation increased from 0 to 0.33%. However, the source of Met did not affect the FI (Table 2). Feed to gain ratio (F:G) was not affected by Met supplementation. However, birds with L-Met tended to have enhanced (P = 0.053) F: G from 1.70 (an average of 0.17 and 0.33% DL-Met) to 1.63 (an average of 0.17 and 0.33% L-Met) than those with DL-Met (Table 2).
Relative Bioavailability
The WG of birds were fitted (P < 0.01) to linear models (Figure 1). The WG of birds linearly increased (P < 0.05) as birds received diets with increasing levels of Met. The slopes of 2 linear models for WG were 1078 ± 294 for L-Met and 674 ± 290 for DL-Met indicating that increase of WG of L-Met was 160.2 ± 60.3% of that of DL-Met. Values of 95% confidence interval were from 33.6 to 286.7%.
Figure 1.
Weight gain (WG) of turkey poults with increasing intake levels of either supplemental DL-Met or L-Met from d 0 to 28. The overall model for WG acceptably fitted (P < 0.01) the observations (x1= intake of supplemental of DL-Met, and x2 = intake of supplemental of L-Met). In the multilinear regression analysis, x-axis represents supplemental Met intake (g/d). Intercept (693) represents WG achieved with the basal diet. Increase of WG of L-Met was 160.2% of that of DL-Met as calculated by [Slope of L-Met (1078)/Slope of DL-Met (674)] × 100. Values in brackets indicated the 95% confidence interval.
Malondialdehyde
There was no difference in duodenal MDA concentrations among treatments on d 7 (Table 3). On d 28, turkey poults fed a diet supplemented with either 0.33% L-Met or 0.33% DL-Met had reduced (P < 0.05) duodenal MDA concentrations compared with those with the BD (Table 3). There was no difference in duodenal MDA concentrations between 0.33% DL-Met and 0.33% treatments.
Table 3.
Redox status in the duodenum of turkey poults fed diets with supplemental methionine.1
| Supplemental DL-Met, % | Supplemental L-Met, % | ||||
|---|---|---|---|---|---|
| Item | BD2 | 0.33 | 0.33 | SEM | P value |
| Malondiadehyde, nmol/mg protein | |||||
| d 7 | 3.16 | 3.21 | 3.17 | 0.26 | 0.99 |
| d 28 | 3.29a | 2.52b | 2.42b | 0.14 | < 0.05 |
| Protein carbonyl, nmol/mg protein | |||||
| d 7 | 0.831 | 0.737 | 0.431 | 0.303 | 0.63 |
| d 28 | 0.564 | 0.307 | 0.384 | 0.102 | 0.20 |
| Total antioxidant capacity, U/mg protein3 | |||||
| d 7 | 2712 | 2694 | 2740 | 166 | 0.98 |
| d 28 | 3023A | 2682B | 2751B | 101 | 0.068 |
| Glutathione, nmol/mg protein | |||||
| d 7 | 2.59B | 3.06A | 2.94A | 0.131 | 0.072 |
| d 28 | 2.52 | 1.95 | 2.14 | 0.267 | 0.31 |
a,bMeans within a row with different superscripts differ (P < 0.05).
A,BMeans within a row with different superscripts tend to differ (0.05 ≤ P < 0.10).
1n = 12.
2BD = basal diet.
3Unit (U) represents 1 umol/L copper reducing equivalents per mg of protein in duodenal mucosa.
There was no difference in hepatic MDA concentration among treatments on d 7 (Table 4). On d 28, turkey poults fed a diet supplemented with 0.33% L-Met tended to have reduced (P = 0.094, 1.32 to 1.16 nmol/mg protein) concentrations of MDA than those with the BD (Table 4).
Table 4.
Redox status in the liver of turkey poults fed diets with supplemental methionine.1
| Supplemental DL-Met, % | Supplemental L-Met, % | ||||
|---|---|---|---|---|---|
| Item | BD2 | 0.33 | 0.33 | SEM | P value |
| Malondiadehyde, nmol/mg protein | |||||
| d 7 | 1.31 | 1.33 | 1.44 | 0.09 | 0.16 |
| d 28 | 1.32A | 1.27A,B | 1.16B | 0.05 | 0.094 |
| Protein carbonyl, nmol/mg protein | |||||
| d 7 | 2.18 | 1.98 | 2.38 | 0.37 | 0.63 |
| d 28 | 4.09A | 3.00B | 2.84B | 0.36 | 0.065 |
| Total antioxidant capacity, U/mg protein3 | |||||
| d 7 | 2179 | 2101 | 2014 | 138 | 0.68 |
| d 28 | 1967 | 1883 | 2094 | 141 | 0.51 |
| Glutathione, nmol/mg protein | |||||
| d 7 | 2.80B | 3.21A,B | 3.45A | 0.201 | 0.092 |
| d 28 | 2.70 | 2.70 | 2.44 | 0.160 | 0.41 |
A,BMeans within a row with different superscripts tend to differ (0.05 ≤ P < 0.10).
1n = 12.
2BD = basal diet.
3Unit (U) represents 1 umol/L copper reducing equivalents per mg of protein in hepatic mucosa.
Protein Carbonyl
There was no difference in duodenal PC concentrations among treatments on d 7 and 28 (Table 3). Supplemental Met did not affect PC concentrations in the hepatic tissue on d 7 (Table 4). On d 28, turkey poults fed a diet supplemented with 0.33% DL-Met or 0.33% L-Met tended to have lower (P = 0.065) PC concentrations in the hepatic tissue than those with the BD (Table 4).
Total Antioxidant Capacity
There was no difference in duodenal TAC concentrations among treatments on d 7 (Table 3). On d 28, turkey poults fed diets supplemented with 0.33% DL-Met or 0.33% L-Met tended to have lower (P = 0.068) duodenal TAC concentrations than those with the BD (Table 3). In the hepatic tissue, TAC concentration was not affected by the sources and concentrations of Met on d 7 and 28 (Table 4).
Glutathione
Turkey poults fed a diet supplemented with 0.33% DL-Met or 0.33% L-Met tended to have greater (P = 0.072) duodenal GSH concentrations compared with those with the BD on d 7 (Table 3). There was no difference in duodenal GSH concentrations between 0.33% DL-Met and 0.33% L-Met treatments on d 7. On d 28, there was no difference in duodenal GSH concentrations among treatments (Table 3).
Turkey poults fed a diet supplemented with 0.33% L-Met tended to have increased (P = 0.092, 2.80 to 3.45 nmol/mg protein) hepatic GSH concentrations than those with the BD on d 7 (Table 4). On d 28, there was no difference in hepatic GSH concentrations among treatments.
Small Intestinal Morphometry
There was no difference in duodenal and jejunal morphology between turkey poults fed a diet supplemented with 0.33% DL-Met or 0.33% L-Met on d 7 and 28 (Table 5).
Table 5.
Duodenal and jejunal morphometry of turkey poults fed diets with supplemental methionine.1
| Supplemental DL-Met, % | Supplemental L-Met, % | ||||
|---|---|---|---|---|---|
| Item | BD2 | 0.33 | 0.33 | SEM | P value |
| Villus height, μm | |||||
| Duodenum, d 7 | 1224 | 1254 | 1232 | 100 | 0.99 |
| Duodenum, d 28 | 2267 | 2325 | 2430 | 74 | 0.22 |
| Jejunum, d 7 | 456 | 536.8 | 501.6 | 69.6 | 0.57 |
| Jejunum, d 28 | 1055 | 997.2 | 1099 | 64 | 0.65 |
| Villus width, μm | |||||
| Duodenum, d 7 | 141 | 139.2 | 138.9 | 8.1 | 0.55 |
| Duodenum, d 28 | 202 | 184.0 | 187.3 | 10.8 | 0.17 |
| Jejunum, d 7 | 79 | 90.9 | 88.7 | 5.8 | 0.39 |
| Jejunum, d 28 | 117 | 120.3 | 141.0 | 14.1 | 0.56 |
| Crypt depth, μm | |||||
| Duodenum, d 7 | 109 | 100.5 | 111.9 | 5.3 | 0.36 |
| Duodenum, d 28 | 143 | 162.9 | 150.7 | 11.7 | 0.42 |
| Jejunum, d 7 | 92 | 105.8 | 108.1 | 5.1 | 0.11 |
| Jejunum, d 28 | 121 | 124.1 | 128.2 | 6.8 | 0.81 |
| VH: CD3 | |||||
| Duodenum, d 7 | 11.3 | 12.46 | 11.09 | 0.98 | 0.61 |
| Duodenum, d 28 | 16.0 | 14.91 | 16.23 | 1.23 | 0.48 |
| Jejunum, d 7 | 5.0 | 5.10 | 4.89 | 0.69 | 0.57 |
| Jejunum, d 28 | 9.1 | 8.16 | 8.67 | 0.51 | 0.60 |
1n = 12.
2BD = basal diet.
3VH: CD = villus height: crypt depth.
DISCUSSION
This study was designed to study the effects of L-Met on growth performance and redox status of turkey poults compared with the use of conventional DL-Met. Methionine supplementation from 0 to 0.33% to a SCAA deficient basal diet (60% SCAA requirement) improved growth performance of turkey poults. The relative bioavailability of L-Met was calculated as 160% for overall WG. These results indicate that turkey poults required 160 units of DL-Met to achieve the overall WG that were produced by 100 units of L-Met. These results support the original hypothesis that L-Met is better utilized by turkey poults compared with DL-Met. This finding agrees with Noll et al. (1984), who indicated that young turkeys fed with L-Met have 131% better BW than that of DL-Met. Noll et al. (1984) evaluated growth performance of turkey at d 7 to 21 of age, but turkey poults at d 0 to 28 of age were used in this study. In young chicken at d 0 to 21 of age, chicks fed with L-Met have about 140% better ADG than that of DL-Met (Shen et al., 2015). In young pigs at d 26 to 46 of age, nursery pigs fed with L-Met have 144% better ADG than that of DL-Met (Shen et al., 2014). In this study, the greater RBA in WG of turkey poults fed a diet supplemented with L-Met than DL-Met are speculated as one of the major reasons for the difference in F:G.
Amino acids play an important role in protein synthesis, cell signaling, antioxidative functions, and immune functions during AA metabolism in the gut (Stoll et al., 1998; Shoveller at al., 2003). One-third of dietary intake of essential AA is removed in the first pass metabolism by the gut. Among AA, Met is the first limiting AA in diets for poultry and one of the extensively used AA by the gut (Stoll et al., 1998). Shen et al. (2015) demonstrated that the first pass metabolism of Met by the gut affect its redox status and development in broiler chicken. In this study, the first pass metabolism of Met by the gut of turkey improved its redox status by decreasing concentrations of MDA, and potentially increasing GSH in duodenal mucosa regardless of Met sources. L-methionine is a precursor of L-Cys, which plays a key role in maintaining protein biosynthesis and redox status. Thus, the L-Met serves as an indirect precursor of GSH (through Cys), taurine, and inorganic S, and these are major cellular antioxidants (Brosnan and Brosnan, 2006). Therefore, early supplementation of Met to turkey poults enhanced the redox status in duodenum even though these benefits did not affect the gut morphology.
In the liver, turkey poults fed a diet supplemented with either 0.33% L-Met or 0.33% DL-Met tended to have decreased MDA and PC, and increased GSH compared with turkey fed a Met deficient diet. This confirms that supplementation of either form of 0.33% Met improved the metabolic function of Met in the liver. Notably, turkey poults fed a diet supplemented with 0.33% L-Met had reduced MDA and increased GSH compared with turkey poults with 0.33% DL-Met. One possible explanation for the enhanced redox effect with L-Met compared with DL-Met could be due to immediate conversion of L-Met to Cys for GSH synthesis whereas D-Met had to be converted to L-Met in the cytoplasm of hepatocytes (London and Gabel, 1988; Levine et al., 1996; Hasegawa et al., 2005; Luo and Levine, 2009). Additionally, L-Met reacts readily with a various ROS to form Met sulfoxide (Moskovitz et al., 2001; Cudic et al., 2016). Then, Met sulfoxide reductases catalyze a reduction of L-Met sulfoxide back to L-Met, consequently scavenging the ROS (Luo and Levine, 2009; Moskovitz et al., 2016). D-methionine can also be oxidized to Met sulfoxide. However, a study has shown Met sulfoxide from D-Met is not effectively used by Met sulfoxide reductase and could not readily be reduced back to D-Met in an animal body (Stegink et al., 1986; Friedman and Gumbmann, 1988). It has been shown that D-Met is less effective antioxidant in the liver of poultry because it needs to be converted to L-Met (Saunderson, 1985). Therefore, L-Met may be better metabolized and served as a more efficient substrate for antioxidant functions than DL-Met in the liver.
In conclusion, dietary supplementation of 0.33% Met to a diet with S containing AA meeting 60% of the NRC requirement enhanced WG, FI, and redox status by reducing oxidative stress in the gut and liver of turkey poults during the first 28 d of age. Use of L-Met tended to enhance F:G and was more effective in reducing oxidative stress and increasing GSH in the liver compared with the use of DL-Met. Consequently, providing sufficient Met is important to keep reduced oxidative stress status in the gut and liver of turkey poults and the use of L-Met as a source of Met replacing DL-Met seems to be beneficial to turkey poults during the first 28 d of age.
Footnotes
Financial support from North Carolina Agricultural Foundation (Raleigh, NC) and CJ CheilJedang Co. (Seoul, Korea)
REFERENCES
- Baker D. H. 2006. Comparative species utilization and toxicity of sulfur amino acids. J. Nutr. 136:1670S–1675S. [DOI] [PubMed] [Google Scholar]
- Baker D. H. 2009. Advances in protein-amino acid nutrition of poultry. Amino acids. 37:29–41. [DOI] [PubMed] [Google Scholar]
- Baker D. H., Boebel K. P.. 1980. Utilization of the D- and L-isomers of methionine and methionine hydroxyl analogue as determined by chick bioassay. J. Nutr. 110:959–964. [DOI] [PubMed] [Google Scholar]
- Brosnan J. T., Brosnan M. E.. 2006. The sulfur-containing amino acids: An overview. J. Nutr. 136:1636S–1640S. [DOI] [PubMed] [Google Scholar]
- Cudic P., Joshi N., Sagher D., Williams B. T., Stawikowski M. J., Weissbach H.. 2016. Identification of activators of methionine sulfoxide reductases A and B. Biochem. Biophys. Res. Commun. 469:863–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner J. J., Knight C. D.. 1984. Coversion of 2-hydroxy-4-(methylthio)butanoic acid to L-methionine in the chick: a stereospecific pathway. J. Nutr. 114:1716–1723. [DOI] [PubMed] [Google Scholar]
- Fernandez S. R., Aoyagi S., Han Y., Parsons C. M., Baker D. H.. 1994. Limiting order of amino acids in corn and soybean meal for growth of the chick. Poult. Sci. 73:1887–1896. [DOI] [PubMed] [Google Scholar]
- Finkelstein J. D. 1990. Methionine metabolism in mammals. J. Nutr. Biochem. 1:228–237. [DOI] [PubMed] [Google Scholar]
- Friedman M., Gumbmann M. R.. 1988. Nutritional value and safety of methionine derivatives, isomeric dipeptides and hydroxyl analogs in mice. J. Nutr. 118:388–397. [DOI] [PubMed] [Google Scholar]
- Hasegawa H., Shinohara Y., Akahane K., Hashimoto T.. 2005. Direct detection and evaluation of conversion of D-methionine into L-methionine in rats by stable isotope methodology. J. Nutr. 7:209–225. [DOI] [PubMed] [Google Scholar]
- Ji F., McGlone J., Kim S. W.. 2006. Effects of dietary humic substances on pig growth performance, carcass characteristics, and ammonia emission. J. Anim. Sci. 84:2482–2490. [DOI] [PubMed] [Google Scholar]
- Kim S. W., Easter R. A.. 2001. Nutritional value of fish meals in the diet for young pigs. J. Anim. Sci. 79:1829–1839. [DOI] [PubMed] [Google Scholar]
- Levine R. L., Mosoni L., Berlett B. S., Stadtman E. R.. 1996. Methionine residues as endogenous antioxidants in proteins. Proc. Natl. Acad. Sci. USA 93:15036–15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littell R., Henry P., Lewis A., Ammerman C.. 1997. Estimation of relative bioavailability of nutrients using SAS procedures. J. Anim. Sci. 75:2672–2683. [DOI] [PubMed] [Google Scholar]
- London R. E., Gabel S. A.. 1988. A deuterium surface coil NMR study of the metabolism of D-methionine in the liver of the anesthetized rat. Biochemistry. 27:7864–7869. [DOI] [PubMed] [Google Scholar]
- Luo S., Levine R. L.. 2009. Methionine in proteins defends against oxidative stress. FASEB J. 23:464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskovitz J., Du F., Bowman C. F., Yan S. S.. 2016. Methionine sulfoxide reductase A (MsrA) affects beta-amyloid solubility and mitochondrial function in mouse model of Alzheimer's disease. Am. J. Physiol. Endocrinol. Metab. 310:E388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskovitz J., Bar-Noy S., Williams W. M., Requena J., Berlett B. S., Stadtman E. R.. 2001. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl. Acad. Sci. USA 98:12920–12925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council 1994. Nutrient Requirements of Poultry. 9th ed.National Academy of Sciences—NRC: Washington, DC. [Google Scholar]
- Noll S. L., Waibel P. E., Cook R. D., Witmer J. A.. 1984. Biopotency of methionine sources for young turkeys. Poult. Sci. 63:2458–2470. [Google Scholar]
- Pack M., Hoehler D., Lemme A.. 2003. Economic assessment of amino acid responses in growing poultry. Page 459–484 in Amino acids in animal nutrition. D’mello J. P. F., ed. CABI pubishing: London, UK. [Google Scholar]
- Saunderson C. L. 1985. Comparative metabolism of L-methionine, DL-methionine and DL-2-hydroxy 4 methylthiobutanoic acid by broiler chicks. Br. J. Nutr. 54:621–633. [DOI] [PubMed] [Google Scholar]
- Shen Y. B., Weaver A. C., Kim S. W.. 2014. Effect of feed grade-methionine on growth performance and gut health in nursery pigs compared with conventional-methionine. J. Anim. Sci. 92:5530–5539. [DOI] [PubMed] [Google Scholar]
- Shen Y. B., Voilqué G., Kim J., Odle J., Kim S. W.. 2012. Effects of increasing tryptophan intake on growth and physiological changes in nursery pigs. J. Anim. Sci. 90:2264–2275. [DOI] [PubMed] [Google Scholar]
- Shen Y. B., Ferket P. R., Park I., Malheiros R. D., Kim S. W.. 2015. Effects of feed grade -methionine on intestinal redox status, intestinal development, and growth performance of young chickens compared with conventional -methionine. J. Anim. Sci. 93:2977–2986. [DOI] [PubMed] [Google Scholar]
- Shoveller A. K., Brunton J. A., House J. D., Pencharz P. B., Ball R. O.. 2003. Dietary cysteine reduces the methionine requirement by an equal proportion in both parenterally and enterally fed piglets. J. Nutr. 133:4215–4224. [DOI] [PubMed] [Google Scholar]
- Stegink L. D., Bell E. F., Filter L. J. Jr., Ziegler E. E., Andersen D. W., Seligson F. H.. 1986. Effects of equimolar doses of L-methionine, D-methionine and L-methionine-dl-sulfoxide on plasma and urinary amino acid levels in normal adult humans. J. Nutr. 116:1185–1192. [DOI] [PubMed] [Google Scholar]
- Stoll B., Henry J., Reeds P. J., Yu H., Jahoor F., Burrin D. G.. 1998. Catabolism dominates the first-pass intestinal metabolism of dietary essential amino acids in milk protein-fed piglets. J. Nutr. 128:606–614. [DOI] [PubMed] [Google Scholar]
- Thwaites D. T., Anderson C. M.. 2007. Deciphering the mechanisms of intestinal imino (and amino) acid transport: the redemption of SLC36A1. Biochim. Biophys. Acta. 1768:179–197. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Flowers W., Saraiva A., Yeum K.-J., Kim S. W.. 2013. Effect of social ranks and gestation housing systems on oxidative stress status, reproductive performance, and immune status of sows. J Anim Sci. 91:5848–5858. [DOI] [PubMed] [Google Scholar]