FIG 4 .
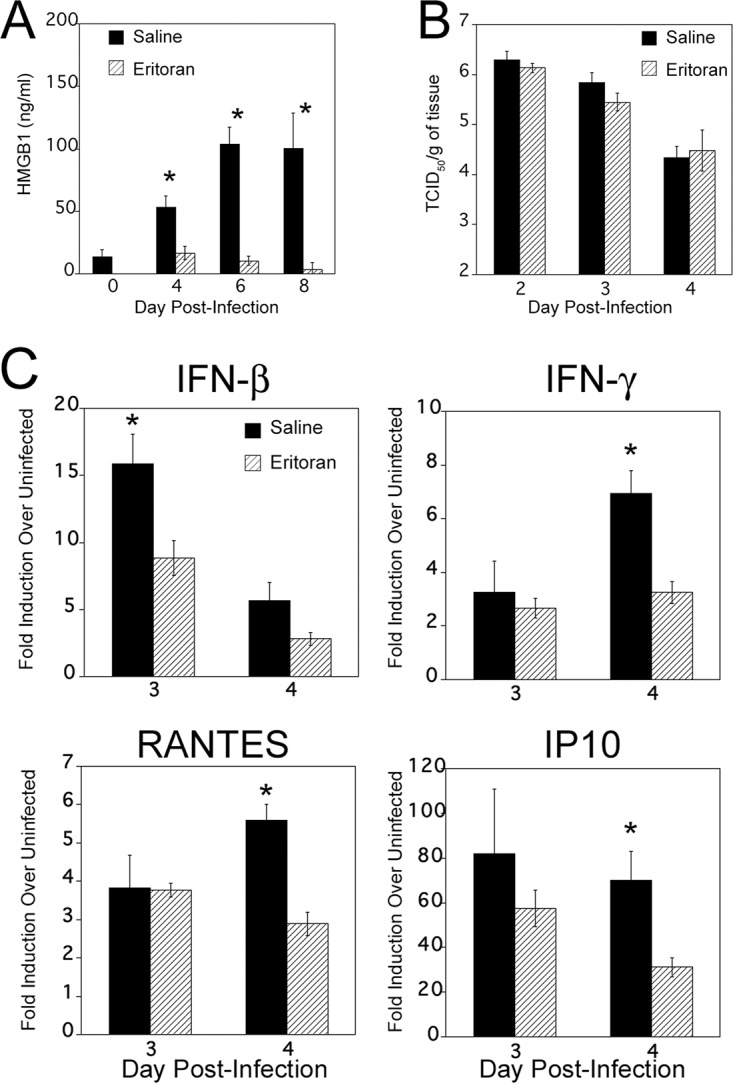
Eritoran treatment blocks influenza B virus-induced serum HMGB1 release in vivo. Cotton rats were infected i.n. with 1 × 106 TCID50 of influenza B virus on day 0. On day 2 p.i., animals were treated with either 200 μl of saline (mock) or 37.3 mg/kg of Eritoran given by the retro-orbital (R.O.) route once daily until day 6 p.i. (A) Serum samples were collected on the indicated days p.i., and HMGB1 levels were measured. Results are from two independent experiments. The numbers of animals per treatment group were 10 (day 0), 20 (4 days p.i.), 15 (6 days p.i.), and 10 (8 days p.i.). Values for saline-treated animals that were significantly different (P less than 0.05) from the value for day 0 or Eritoran-treated HMGB1 are indicated by an asterisk. (B) Eritoran treatment does not affect virus replication in vivo. Animals were infected and treated as described above for panel A. On days 2, 3, and 4 p.i., animals from both saline- and Eritoran-treated groups were sacrificed 4 h after treatment, and nose tissues were collected for virus titration. There were five rats in each group. (C) Relative gene expression profile of IFN-β, IFN-γ, IP10, and RANTES in the lung tissues of saline- or Eritoran-treated, influenza B virus-infected cotton rats. Groups of cotton rats (five animals in each group) were euthanized on the indicated day p.i., and the lungs were collected for cytokine mRNA analysis by qRT-PCR. The results were calculated as fold induction for each cytokine over the level of expression in uninfected animals and expressed as geometric means ± SEMs. Values for saline-treated animals that are significantly different (P < 0.05) from the values for Eritoran-treated animals are indicated by an asterisk.
