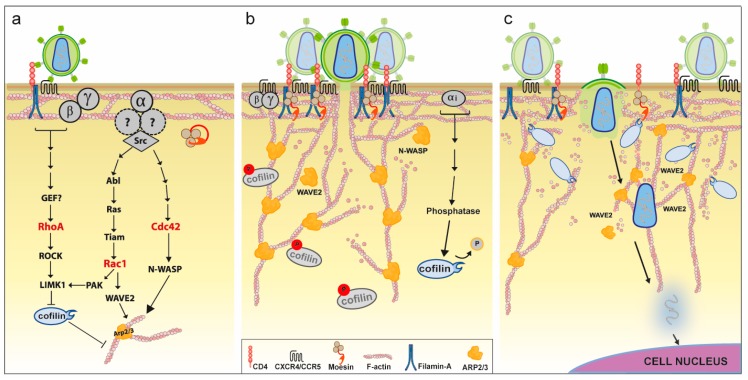
Figure 2.
Hypothetical model of the role of manipulated actin dynamics for inbound HIV infection in T-cells. (a) Early signaling events. Binding of Env to CD4 and coreceptor molecules (CXCR4/CCR5) initiates signaling via the receptor-coupled G-protein. Gαi/Gαq signaling via Src activates both Rac1 and Cdc42, which in turn activate downstream nucleation-promoting factors (NPFs) that stimulate actin polymerization activity by the Arp2/3 complex. Env binding to CD4 also triggers RhoA activation in a filamin-A-dependent manner, and results in moesin activating phosphorylation. Both RhoA and Rac1 lead to cofilin inactivation via phosphorylation by LIMK1. Rho-GTPases are shown in red; (b) receptor capping. Stimulation of Arp2/3 by active NPFs and simultaneous inhibition of cofilin leads to increased cortical actin polymerization. This results in aggregation of CD4 and CXCR4 receptors, which requires linking of these molecules to actin filaments by filamin-A and moesin. Enhanced colocalization of these receptors near Env promotes viral entry (fusion), with actin polymerization participating in fusion-pore formation and expansion. Delayed Env-CXCR4 signaling via Gαi leads to cofilin activating dephosphorylation by a currently undefined mechanism; (c) intracellular transport. After virus–cell fusion, transport of HIV to the nucleus is limited by the dense cortical actin network. Cofilin activation results in local actin depolymerization that allows passage of the viral core through the actin cortex. Transport of the pre-integration complex towards the nucleus is thought to involve Arp2/3-mediated actin polymerization. * Of note, interfering with the Rho-GTPases shown in Red, or any of the molecules depicted in the legend has been reported to impair inbound HIV infection.