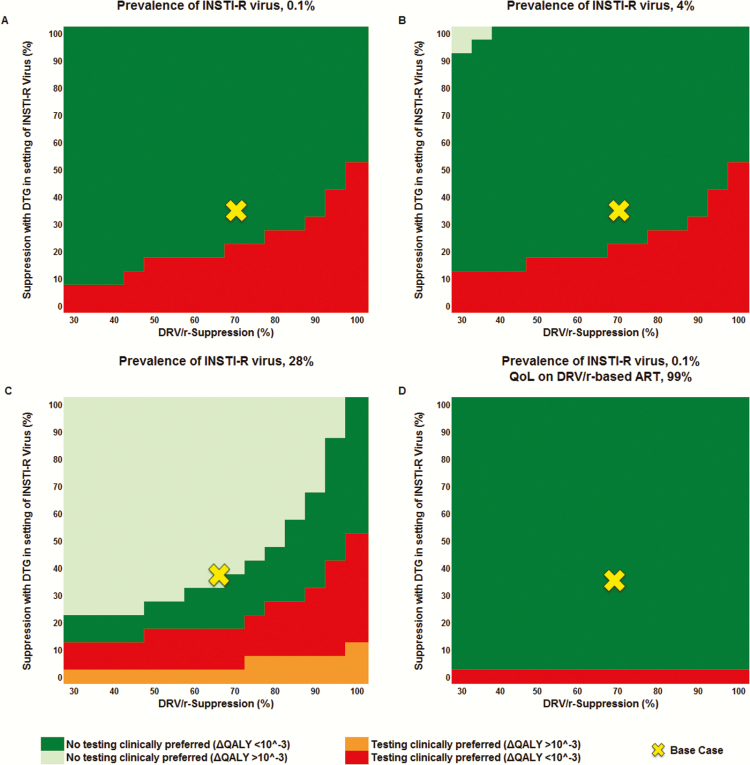
Figure 2.
Multivariate sensitivity analysis of the clinical impact (quality-adjusted life-years [QALYs]) of integrase strand transfer inhibitor (INSTI) resistance testing (IR testing) compared to no IR testing while varying the probability of dolutegravir (DTG) suppression of INSTI-resistant (INSTI-R) virus (vertical axis) and ritonavir-boosted darunavir (DRV/r) suppression (horizontal axis). Prevalence of transmitted INSTI-R virus is 0.1% in the base case (A). IR testing is clinically preferred (red and orange) when DTG suppression of INSTI-R virus is low (bottom) and suppression with DRV/r is high (right); no IR testing is clinically preferred (dark green and light green) when DTG suppression of INSTI-R virus is high (top) and virologic suppression with DRV/r is low (left). A–C, Quality of life (QoL) on DRV/r-based antiretroviral therapy (ART) is equivalent to DTG-based ART. D, QoL on DRV/r-based ART is reduced to 99% of that on DTG-based ART. Beginning at an INSTI-R prevalence of 4%, the no IR testing strategy showed a gain of ≥10–3 QALYs compared to the testing strategy (light green) (B); at an INSTI-R prevalence of 28%, the IR testing strategy resulted in a gain of ≥10–3 QALYs (orange) (C).