Key Points
Among infants hospitalized with bronchiolitis, low serum LL-37 levels were independently associated with intensive care use and longer length-of-stay. Low levels of serum LL-37 were also independently associated with respiratory syncytial virus. By contrast, high LL-37 levels were associated with rhinovirus.
Keywords: bronchiolitis, LL-37, respiratory syncytial virus, microbiota, severity of illness
Abstract
Background
LL-37 is a host defense peptide with antimicrobial and immunomodulatory properties. We examined the relation of serum LL-37 levels to the severity of bronchiolitis and viral etiology.
Methods
We performed a 17-center prospective cohort study in infants hospitalized with bronchiolitis over 3 winters (2011–2014). Site teams collected clinical data, nasopharyngeal aspirates and serum. We used real-time polymerase chain reaction to test nasopharyngeal aspirates for 16 viruses. We tested serum for LL-37. Severity of bronchiolitis was defined by intensive care use and hospital length of stay. Viral etiology was defined as respiratory syncytial virus (RSV) or rhinovirus (RV), including coinfections with other viruses.
Results
The median age of the 1005 enrolled infants was 3 months (interquartile range, 2–6 months). After adjustment for 12 variables, LL-37 levels in the lowest quartile, compared with the highest, were associated both with intensive care use (adjusted odds ratio [aOR], 1.97; P = .01) and longer hospital stay (1.34; P < .001). In separate multivariable models, infants with LL-37 levels in the lowest 3 quartiles, compared with the highest, were more likely to have RSV (eg, aOR, 2.6 [lowest quartile]; P < .001 [all quartiles]). By contrast, infants with the lowest 3 LL-37 quartiles were less likely to have RV (eg, aOR, 0.5 [lowest quartile]; Pall quartiles ≤ .03 [all quartiles]).
Conclusions
In a large multicenter study of infants hospitalized with bronchiolitis, lower levels of serum LL-37 were associated with increased severity of illness. There was also an inverse relationship between LL-37 levels and the most common virus causing bronchiolitis, RSV. These findings highlight the role of LL-37 in the pathogenesis of bronchiolitis.
Although most children with bronchiolitis experience mild symptoms, some have severe illness and require hospitalization. Indeed, bronchiolitis is the leading cause of hospitalization for US infants and accounts for approximately 130000 hospitalizations annually [1, 2]. Respiratory syncytial virus (RSV) is the most common virus causing bronchiolitis [3, 4]; the second most common is rhinovirus (RV) [3, 4]. RSV and RV together account for about 85% of children hospitalized with bronchiolitis [3].
LL-37 is an antimicrobial peptide with antiviral and immunomodulatory effects [5, 6]. In response to infection, neutrophils, macrophages, and bronchial epithelial cells all produce LL-37, a cleavage product of human cathelicidin antimicrobial peptide [5]. Previous studies have demonstrated that lower levels of serum LL-37 in patients undergoing hemodialysis are associated with increased mortality rates [7]. In human and laboratory studies, LL-37 has been shown to have antiviral activity against RSV [8–10], and reduce RV replication [11].
Mansbach et al [12] previously reported in a pilot study of 82 children with bronchiolitis that serum human cathelicidin antimicrobial peptide levels less than the median were associated with more severe bronchiolitis (defined by hospitalization for ≥24 hours) and increased frequency of RSV infection (compared with RV). More recently, Hasegawa et al [13] reported that infants with lower levels of serum LL-37 and a Haemophilus-dominant airway microbiota are more likely to require intensive care; that analysis specifically examined the interaction between LL-37 and Haemophilus dominance but not the direct association between LL-37 and either severity of illness or viral etiology. This knowledge gap and the promise of LL-37 as an inducible host defense peptide with beneficial clinical effects [14] led us to reexamine our previous findings in a much larger sample of infants hospitalized with bronchiolitis.
METHODS
Study Design
We performed a prospective cohort study of hospitalized infants (aged <1 year) with an attending physician’s diagnosis of bronchiolitis at 17 hospitals across the United States. Site teams enrolled hospitalized infants from 1 November to 30 April for 3 consecutive years (2011–2014). This study is called the 35th Multicenter Airway Research Collaboration, or MARC-35. MARC is a program of the Emergency Medicine Network (EMNet) (www.emnet-usa.org). One of the short-term goals of MARC-35 is to better understand the factors associated with severity of illness at the time of hospitalization. Its long-term goals are to determine factors associated with recurrent wheezing of childhood by age 3 years and eventual asthma at age 6 years.
We included families with children aged <1 year, and with a gestational age ≥32 weeks, who were hospitalized with an attending physician diagnosis of bronchiolitis, as defined by the Academy of Pediatrics; participating families agreed to long-term follow-up with a stable address, phone number, e-mail, and primary care provider and provided informed consent.
We excluded families who (1) did not agree to the collection of nasopharyngeal aspirate or blood; (2) agreed to participate >24 hours after admission to the ward or intensive care unit; (3) already met the definition of the primary outcome, recurrent wheezing (ie, 2 oral steroid courses in 6 months or 4 episodes of wheezing in past year); or (4) had known chronic medical conditions, such as heart-lung disease, immunodeficiency, or immunosuppression. All patients were treated at the discretion of the treating clinician. The institutional review board at each of the 17 participating hospitals approved the study.
Data Collection
Investigators conducted structured interviews to collect demographic characteristics, as well as detailed medical, environmental, nutritional, and family histories. Site teams also reviewed emergency department and hospital charts to collect details of the hospitalization.
Blood and Nasopharyngeal Aspirate Collection
All site teams collected blood samples as soon as possible during the hospitalization. All nasopharyngeal aspirates were collected within 24 hours of hospitalization using the same protocol and equipment (eg, sample traps and suction catheters from Medline Industries) used in previous studies [3, 15].
LL-37 and 25-Hydroxyvitamin D Assays
We quantified serum LL-37 concentrations using the Hycult Biotech Human LL-37 enzyme-linked immunosorbent assay. We quantified 25-hydroxyvitamin D (25[OH])D) using the Abbott ARCHITECT 25-OH Vitamin D chemiluminescent microparticle immunoassay. Bioavailable 25(OH)D was calculated using 25(OH)D, albumin, and vitamin D-binding protein values [16, 17]. Vitamin D–binding protein values were calculated using the Quantikine Human Vitamin D Binding Protein Immunoassay.
Polymerase Chain Reaction Assays
Polymerase chain reaction assays for 16 viruses were conducted at Baylor College of Medicine, as described elsewhere [18–20]. We tested all samples for RSV types A and B, RV, parainfluenza virus types 1, 2 and 3, influenza virus types A and B, human metapneumovirus, coronaviruses NL-63, HKU1, OC43 and 229E, adenovirus, human bocavirus type 1, and enterovirus.
16S Ribosomal RNA Gene Sequencing and Compositional Analysis
To examine the structure of microbiota, we also sequenced the 16S ribosomal RNA gene V4 region of the nasopharyngeal aspirates on the Illumina MiSeq platform. Only microbiota data with sufficient sequence depth (ie, rarefaction cutoff of 2128 readings per sample) were used. As described elsewhere [21], we generated 4 distinct microbiota clusters using an unbiased clustering approach (ie, partitioning around medoids), weighted UniFrac distances [22], and the average silhouette score [23]. The 4 airway microbiota clusters in the nasopharyngeal aspirate were: Haemophilus dominant, Moraxella dominant, Streptococcus dominant, and mixed.
Primary Exposure and Outcome Measures
For the present analysis, we categorized the serum LL-37 levels into quartiles: lowest (<34 ng/mL), second lowest (34–45.9 ng/mL), second highest (46–59.9 ng/mL), and highest (≥60 ng/mL). The primary outcome was severity of illness, defined as intensive care use (ie, admission to an intensive care unit or use of continuous positive airway pressure and/or intubation) at any time during the index hospitalization [15, 24]. The secondary measure of severity was hospital length of stay (LOS) as a count variable. The virus outcomes were RSV and RV, which included virus-only infections (ie, RSV only and RV only) and any combination of viral coinfection with RSV and RV, including the specific RSV-RV coinfection.
Statistical Methods
Across the LL-37 quartiles, we compared patient characteristics and hospital course using χ2 test or Kruskal-Wallis test, as appropriate. To examine the association of LL-37 quartiles with the outcomes, we constructed 3 logistic regression models, 1 model for the primary clinical outcome (intensive care use) and 2 models for the virus outcomes (RSV and RV positive), as well as Poisson regression models for the secondary clinical outcome of hospital LOS. For each outcome, the first model is unadjusted and the second is a 2-level mixed-effects multivariable regression model that accounts for patient clustering at the hospital level. All of the multivariable models adjust for 12 factors: age, sex, race/ethnicity, prematurity, history of breathing problems, daycare attendance, siblings at home, smoke exposure at home, antibiotic use before the index hospitalization, corticosteroid use before the index hospitalization, viral etiology (excluded when viruses were used as the outcome), and airway microbiota clusters.
We chose these potential confounders based on clinical plausibility and a priori knowledge [3, 15, 24–26]. We did not adjust for markers of severity (eg, vital signs) or measures of vitamin D—for example, 25(OH)D levels or bioavailable 25(OH)D) [27, 28]—because these were considered intermediate or upstream factors, rather than confounders, for the associations of interest. Infant weight was not included because it is strongly correlated with age. We also fit locally weighted scatterplot smoothing (LOESS) plots that show the nonlinear relationship between LL-37 as a continuous variable and the 2 severity outcomes. Analyses used R version 3.3.1 with the lme4 package for the mixed-effects models and the phyloseq package [29]. All P values were 2 tailed, with differences considered statistically significant at P < .05.
RESULTS
Of the 1016 children enrolled in MARC-35, 1005 had complete data and comprised the analytic cohort. The median age at hospitalization was 3 months (interquartile range [IQR], 2–6 months), 60% were male, and 43% were non-Hispanic white. Overall, 161 (16%) infants hospitalized for bronchiolitis required intensive care use. The overall median hospital LOS was 2 days (IQR, 1–3). The infants included 813 (81%) infants with RSV infection, 211 (21%) with RV infection, and 120 (12%) with specific RSV-RV coinfection. Together, RSV and RV accounted for 90% of the bronchiolitis hospitalizations.
Overall, the median level of serum LL-37 was 46 ng/mL (IQR, 34–60 ng/mL). Table 1 shows patient characteristics, clinical variables, virology, airway microbiota clusters, and laboratory data by LL-37 quartile. In general, older infants who had attended daycare had significantly higher levels of LL-37. There were no differences in either serum 25(OH)D or bioavailable 25(OH)D by quartile of LL-37 in this acutely ill population.
Table 1.
Patient Variables in 1005 Infants Hospitalized for Bronchiolitis by Quartiles of Serum LL-37 Levels
| Patients by Quartile of Serum LL-37 Levels, No. (%)a | P Value | ||||
|---|---|---|---|---|---|
| Lowest Quartile (<34 ng/mL; n = 23) | Second-Lowest Quartile (34–45.9 ng/mL; n = 235) | Second-Highest Quartile (46–59.9 ng/mL; n = 265) | Highest Quartile (≥60 ng/mL; n = 273) | ||
| Patient characteristics | |||||
| Age median (IQR), mo | 2.2 (1.4–4.7) | 2.9 (1.6–5.6) | 3.6 (1.9–6.0) | 3.8 (2.2–6.7) | <.001b |
| Age group | |||||
| <2 mo | 106 (46) | 76 (32) | 69 (26) | 58 (21) | <.001b |
| 2–5.9 mo | 81 (35) | 107 (46) | 129 (49) | 133 (49) | |
| 6–12 mo | 45 (19) | 52 (22) | 67 (25) | 82 (30) | |
| Male sex | 145 (63) | 137 (58) | 159 (60) | 162 (59) | .82 |
| Race/ethnicity | |||||
| Non-Hispanic white | 115 (50) | 94 (40) | 111 (42) | 108 (40) | .47 |
| Non-Hispanic black | 48 (21) | 52 (22) | 62 (23) | 71 (26) | |
| Hispanic | 59 (25) | 79 (34) | 83 (31) | 85 (31) | |
| Other | 10 (4) | 10 (4) | 9 (3) | 9 (3) | |
| Parental history of asthma | 83 (36) | 83 (35) | 84 (32) | 91 (33) | .77 |
| Maternal smoking during pregnancy | 35 (15) | 44 (19) | 23 (9) | 42 (16) | .01b |
| Cesarean delivery | 82 (36) | 83 (36) | 93 (36) | 85 (32) | .68 |
| Prematurity (32–37 wk) | 48 (21) | 44 (19) | 48 (18) | 43 (16) | .55 |
| Breathing problems before index hospitalizationc | 40 (17) | 40 (17) | 54 (20) | 69 (25) | .07 |
| History of eczema | 39 (17) | 26 (11) | 35 (13) | 46 (17) | .18 |
| Ever attended daycare | 39 (17) | 53 (23) | 55 (21) | 83 (30) | .003b |
| Sibling in household | 192 (83) | 184 (78) | 214 (81) | 209 (77) | .33 |
| Mostly breastfed in first 3 mo of life | 102 (52) | 93 (45) | 106 (46) | 118 (49) | .52 |
| Smoke exposure at home | 27 (12) | 41 (17) | 40 (15) | 45 (17) | .31 |
| Antibiotic use before index hospitalization | 72 (31) | 65 (28) | 89 (34) | 86 (32) | .56 |
| Corticosteroid use before index hospitalization | 30 (13) | 35 (15) | 36 (14) | 45 (17) | .68 |
| Clinical presentation | |||||
| Weight at presentation, median (IQR), kg | 5.5 (4.4–7.2) | 5.8 (4.5–7.6) | 6.4 (5.0–7.8) | 6.6 (5.1–8.1) | <.001b |
| Respiratory rate at presentation, median (IQR), respirations per min | 50 (40–60) | 48 (40–60) | 48 (40–60) | 50 (40–60) | .72 |
| Oxygen saturation at presentation | .01b | ||||
| <88% | 20 (9) | 13 (6) | 6 (2) | 16 (6) | |
| 88%-89% | 4 (2) | 8 (4) | 9 (4) | 13 (5) | |
| 90%-93% | 42 (18) | 27 (12) | 35 (14) | 49 (18) | |
| ≥94% | 162 (71) | 180 (79) | 210 (81) | 189 (71) | |
| Received antibiotics during prehospitalization visit | 57 (25) | 35 (15) | 43 (16) | 40 (15) | .01b |
| Received corticosteroids (systemic or inhaled) during prehospitalization visit | 22 (10) | 24 (10) | 12 (5) | 31 (11) | .03b |
| Virology | .001b | ||||
| Sole RSV infection | 148 (64) | 143 (61) | 158 (60) | 131 (48) | |
| Sole RV infection | 11 (5) | 9 (4) | 10 (4) | 30 (11) | |
| RSV + RV coinfection | 26 (11) | 23 (10) | 36 (14) | 35 (13) | |
| RSV + non-RV pathogens | 24 (10) | 34 (15) | 28 (11) | 27 (10) | |
| RV + non-RSV pathogens | 3 (1) | 5 (2) | 8 (3) | 15 (6) | |
| Neither RSV nor RV | 20 (9) | 21 (9) | 25 (9) | 35 (13) | |
| Nasopharyngeal microbiota profile | .75 | ||||
| Moraxella dominant | 45 (19) | 55 (23) | 64 (24) | 56 (21) | |
| Haemophilus dominant profile | 44 (19) | 41 (17) | 50 (19) | 58 (21) | |
| Streptococcus dominant | 69 (30) | 61 (26) | 80 (30) | 73 (27) | |
| Mixed | 74 (32) | 78 (33) | 71 (27) | 86 (32) | |
| Laboratory data | |||||
| Serum 25-hydroxyvitamin D, median (IQR), ng/mL | 25.9 (16.4–32.2) | 26.8 (19.1–33.4) | 26.6 (19.1–32.8) | 26.8 (19.1–33.7) | .38 |
| Serum bioavailable 25-hydroxyvitamin D, median (IQR), ng/mL | 4.23 (2.7–7.1) | 4.41 (3.0–7.2) | 3.9 (2.7–6.6) | 4.2 (3.0–6.2) | .24 |
| Hospital course | |||||
| Intensive care used | 57 (25) | 32 (14) | 38 (14) | 34 (13) | .001b |
| Hospital LOS, median (IQR), d | 2.0 (1.0–4.0) | 2.0 (1.0–3.5) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | .009b |
Abbreviations: IQR, interquartile range; LOS, length of stay; RSV, respiratory syncytial virus; RV, rhinovirus.
aData represent No. (%) of patients unless otherwise indicated. Percentages may not total 100%, because of missingness.
bSignificant at P < .05.
cDefined as a cough that wakes the child at night and/or causes emesis, or wheezing or shortness of breath without cough.
dDefined as admission to intensive care unit and/or use of mechanical ventilation (continuous positive airway pressure and/or intubation during inpatient stay, regardless of location) at any time during the index hospitalization.
LL-37 and Severity of Illness
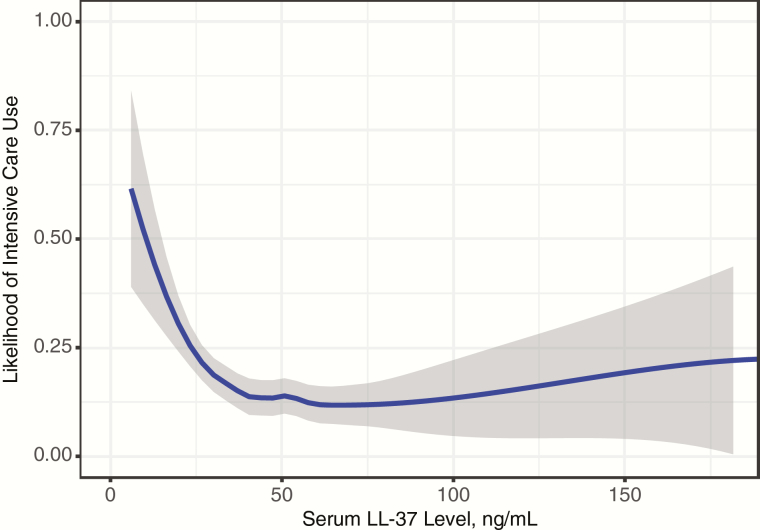
The rate of intensive care use was highest in the infants with the lowest levels of LL-37 versus those with the highest levels (25% vs 13%; unadjusted odds ratio [OR], 2.29; P < .001; Tables 1 and 2). When LL-37 was examined as a continuous variable the inverse relationship between the level of LL-37 and intensive care use remained (Figure 1). Furthermore, after adjustment for 12 factors (see Supplementary Figure S1 for LOESS plot of age and likelihood of intensive care) and clustering by site, the rate of intensive care use was significantly higher in children in the lowest quartile of LL-37, compared with those in the highest quartile (OR, 1.97; P = .01; Table 2). One of the unique factors in the present analysis was our ability to adjust for previously identified airway microbiota clusters [21]. As in previous reports, we found that a Haemophilus-dominant microbiota was significantly associated with intensive care [13, 21].
Table 2.
Unadjusted and Adjusted Associations of Serum LL-37 Levels With Intensive Care Usea
| Serum LL-37 Levels and Covariates | Unadjusted Model | Adjusted Modelb | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Serum LL-37 level by quartile | ||||
| Lowest (<34 ng/mL) | 2.29 (1.44–3.68) | <.001c | 1.97 (1.15–3.37) | .01c |
| Second lowest (34–45.9 ng/mL) | 1.11 (.66–1.86) | .70 | 0.94 (.53–1.67) | .83 |
| Second highest (46–59.9 ng/mL) | 1.18 (.72–1.94) | .52 | 1.05 (.60–1.81) | .87 |
| Highest (≥60 ng/mL) | Reference | … | Reference | … |
| Covariates | ||||
| Age | ||||
| <2 mo | … | … | Reference | … |
| 2–5.9 mo | … | … | 0.34 (.22–.54) | <.001c |
| 6–12 mo | … | … | 0.32 (.18–.59) | <.001c |
| Female (vs male) sex | … | … | 0.89 (.61–1.30) | .56 |
| Race/ethnicity | ||||
| Non-Hispanic white | … | … | Reference | … |
| Non-Hispanic black | … | … | 0.84 (.49–1.43) | .51 |
| Hispanic | … | … | 1.16 (.7–1.92) | .57 |
| Other | … | … | 1.65 (.61–4.49) | .32 |
| Prematurity (32–37 wk) | … | … | 1.36 (.86–2.15) | .19 |
| Breathing problems before index hospitalization | … | … | 1.34 (.79–2.26) | .28 |
| Ever attended daycare | … | … | 0.75 (.42–1.34) | .33 |
| Sibling at home | … | … | 1.24 (.75–2.05) | .39 |
| Smoke exposure at home | … | … | 0.58 (.30–1.09) | .09 |
| Antibiotic use before index hospitalization | … | … | 0.76 (.49–1.19) | .23 |
| Corticosteroid use before index hospitalization | … | … | 1.95 (1.12–3.41) | .02c |
| Virology | ||||
| Sole RSV infection | … | … | Reference | … |
| Sole RV infection | … | … | 0.79 (.35–1.79) | .57 |
| RSV + RV coinfection | … | … | 1.44 (.81–2.54) | .21 |
| RSV + non-RV pathogens | … | … | 1.03 (.55–1.92) | .92 |
| RV + non-RSV Pathogens | … | … | 0.41 (.09–1.85) | .24 |
| Neither RSV nor RV | … | … | 1.19 (.63–2.25) | .60 |
| Nasopharyngeal microbiota profile | ||||
| Moraxella dominant | … | … | Reference | … |
| Haemophilus dominant | … | … | 2.28 (1.25–4.17) | .008c |
| Streptococcus dominant | … | … | 1.57 (.89–2.78) | .12 |
| Mixed | … | … | 1.37 (.79–2.41) | .27 |
Abbreviations: CI, confidence interval; OR, odds ratio; RSV, respiratory syncytial virus; RV, rhinovirus.
aDefined as admission to intensive care unit and/or use of mechanical ventilation (continuous positive airway pressure and/or intubation during inpatient stay, regardless of location) at any time during the index hospitalization.
bMixed-effects logistic regression model adjusting for sites as random effects.
cSignificant at P < .05.
Figure 1.
Locally weighted scatterplot smoothing (LOESS) plot showing the relationship between LL-37 as a continuous variable and the likelihood of intensive care use. Shading around the line represents 95% confidence intervals.
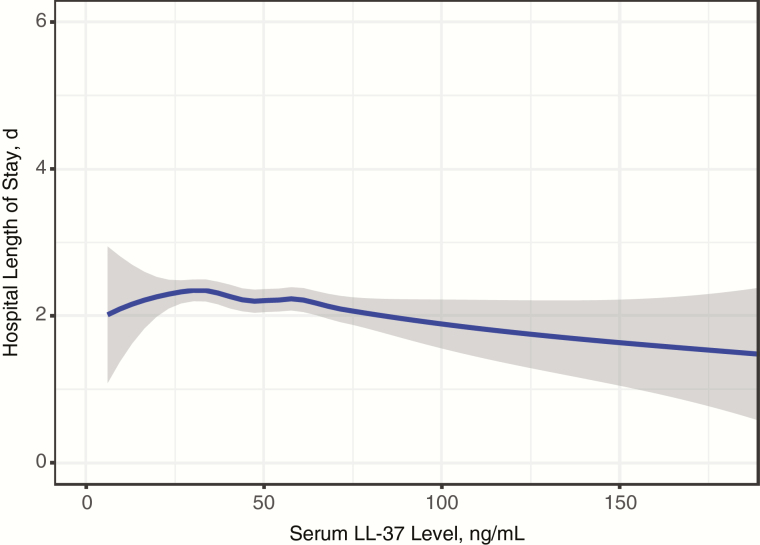
The inverse relationship between serum LL-37 and severity of illness is further supported when hospital LOS is used as the outcome. The hospital LOS was longer in children in the lowest quartile of LL-37 levels, compared with those in the highest quartile (unadjusted rate ratio, 1.52; P < .001; Tables 1 and 3). This inverse relationship is again shown in the LOESS plot (Figure 2). The significant association persisted in the multivariable results. After adjustment for the same 12 factors used in the intensive care model, infants in the lowest and second-lowest quartiles of LL-37 levels had significantly longer LOS than those in the highest quartile (Table 3). The second-highest quartile of LL-37 levels had a nonsignificant association with longer LOS (Table 3).
Table 3.
Unadjusted and Adjusted Associations of Serum LL-37 Levels With Hospital Length of Stay
| Serum LL-37 Levels and Covariates | Unadjusted Model | Adjusted Modela | ||
|---|---|---|---|---|
| RR (95% CI) | P Value | RR (95% CI) | P Value | |
| Serum LL-37 level by quartile | ||||
| Lowest (<34 ng/mL) | 1.52 (1.37–1.69) | <.001b | 1.34 (1.2–1.49) | <.001b |
| Second lowest (34–45.9 ng/mL) | 1.26 (1.13–1.41) | <.001b | 1.16 (1.04–1.3) | .01b |
| Second highest (46–59.9 ng/mL) | 1.11 (1.00–1.23) | .06 | 1.06 (.95–1.18) | .30 |
| Highest (≥60 ng/mL) | Reference | … | Reference | … |
| Covariates | ||||
| Age group | ||||
| <2 mo | … | … | Reference | … |
| 2–5.9 mo | … | … | 0.65 (.59–.71) | <.001b |
| 6–12 mo | … | … | 0.55 (.49–.62) | <.001b |
| Female (vs male) sex | … | … | 0.95 (.88–1.03) | .22 |
| Race/ethnicity | ||||
| Non-Hispanic white | … | … | Reference | … |
| Non-Hispanic black | … | … | 0.84 (.75–.93) | .001b |
| Hispanic | … | … | 1.19 (1.08–1.31) | <.001b |
| Other | … | … | 0.92 (.73–1.14) | .44 |
| Prematurity (32–37 wk) | … | … | 1.23 (1.12–1.35) | <.001b |
| Breathing problems before index hospitalization | … | … | 1.13 (1.02–1.25) | .02b |
| Ever attended daycare | … | … | 1.05 (.95–1.17) | .32 |
| Sibling at home | … | … | 1.05 (.95–1.15) | .32 |
| Smoke exposure at home | … | … | 1.01 (.9–1.13) | .87 |
| Antibiotic use before index hospitalization | … | … | 0.94 (.86–1.02) | .15 |
| Corticosteroid use before index hospitalization | … | … | 1.09 (.97–1.23) | .14 |
| Virology | ||||
| Sole RSV infection | … | … | Reference | … |
| Sole RV infection | … | … | 0.75 (.63–.89) | .001b |
| RSV + RV coinfection | … | … | 0.95 (.85–1.08) | .44 |
| RSV + non-RV pathogens | … | … | 0.95 (.84–1.08) | .46 |
| RV + non-RSV pathogens | … | … | 1.33 (1.1–1.62) | .004b |
| Neither RSV nor RV | … | … | 0.96 (.85–1.1) | .59 |
| Nasopharyngeal microbiota profile | ||||
| Moraxella dominant | … | … | Reference | … |
| Haemophilus dominant | … | … | 1.28 (1.15–1.44) | <.001b |
| Streptococcus dominant | … | … | 1.01 (.91–1.12) | .89 |
| Mixed | … | … | 0.91 (.82–1.02) | .10 |
Abbreviations: CI, confidence interval; RR, rate ratio; RSV, respiratory syncytial virus; RV, rhinovirus.
aMixed-effects Poisson regression model adjusting for sites as random effects.
bSignificant at P < .05.
Figure 2.
Locally weighted scatterplot smoothing (LOESS) plot showing the relationship between LL-37 as a continuous variable and hospital length of stay as a count variable. Shading around the line represents 95% confidence intervals.
LL-37 and Viral Etiology
When we examined the proportions of children with RSV only and RV only by LL-37 quartile (Table 1), there seemed to be a threshold effect. At the highest quartile of serum LL-37 levels, RSV-only infections are less common and RV-only infections are more common, compared with any of the other 3 quartiles (P < .001). This same pattern is seen in both the unadjusted and adjusted models. Compared with the highest quartile of LL-37 levels, infants with LL-37 levels in the 3 lowest quartiles were significantly more likely (OR, ≥2.14; P < .001) to have RSV bronchiolitis (Table 4).The results are consistent even after adjustment for 11 factors and clustering by site (Table 4). One factor we adjusted for was airway microbiota composition, and we found that a Streptococcus-dominant airway microbiota was positively associated with RSV. By contrast, RV infection is more common at the highest levels of LL-37. Compared with the highest quartile of LL-37 levels, infants in the 3 lowest quartiles were significantly less likely (OR, ≤0.62; P = .02) to have RV bronchiolitis (Table 5). The results are consistent even after adjustment for 11 factors and clustering by site (Table 5).
Table 4.
Unadjusted and Adjusted Associations of Serum LL-37 Levels with Respiratory Syncytial Virus Infectiona
| Serum LL-37 Levels and Covariates | Unadjusted Model | Adjusted Modelb | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Serum LL-37 level by quartile | ||||
| Lowest (<34 ng/mL) | 2.41 (1.55–3.81) | <.001c | 2.59 (1.54–4.36) | <.001c |
| Second lowest (34–45.9 ng/mL) | 2.37 (1.53–3.73) | <.001c | 2.41 (1.48–3.92) | <.001c |
| Second highest (46–59.9 ng/mL) | 2.14 (1.42–3.27) | <.001c | 2.3 (1.45–3.65) | <.001c |
| Highest (≥60 ng/mL) | Reference | … | Reference | … |
| Covariates | ||||
| Age group | ||||
| <2 mo | … | … | Reference | … |
| 2–5.9 mo | … | … | 1.27 (.81–1.99) | <.001c |
| 6–12 mo | … | … | 0.72 (.43–1.20) | <.001c |
| Female (vs male) sex | 0.69 (.48–.98) | .04 | ||
| Race/ethnicity | ||||
| Non-Hispanic white | … | … | Reference | … |
| Non-Hispanic black | … | … | 0.6 (.38–.95) | .03c |
| Hispanic | … | … | 0.68 (.44–1.05) | .08 |
| Other | … | … | 1.11 (.40–3.09) | .84 |
| Prematurity (32–37 wk) | … | … | 1.08 (.69–1.69) | .74 |
| Breathing problems before index hospitalization | … | … | 0.36 (.24–.54) | <.001c |
| Ever attended daycare | … | … | 1.86 (1.18–2.91) | .01c |
| Sibling at home | … | … | 0.9 (.58–1.39) | .64 |
| Smoke exposure at home | … | … | 0.89 (.55–1.43) | .62 |
| Antibiotic use before index hospitalization | … | … | 1.04 (.71–1.55) | .83 |
| Corticosteroid use before index hospitalization | … | … | 0.81 (.5–1.31) | .39 |
| Nasopharyngeal microbiota profile | ||||
| Moraxella dominant | … | … | Reference | … |
| Haemophilus dominant | … | … | 0.76 (.47–1.23) | .26 |
| Streptococcus dominant | … | … | 2.68 (1.58–4.56) | <.001c |
| Mixed | … | … | 1.53 (.96–2.44) | .07 |
Abbreviations: CI, confidence interval; OR, odds ratio.
aInfection with respiratory syncytial virus only or with other virus(es).
bMixed-effects logistic regression models adjusting for sites as random effects.
cSignificant at P < .05.
Table 5.
Unadjusted and Adjusted Associations of Serum LL-37 Levels with Rhinovirus Infectiona
| Serum LL-37 Levels and Covariates | Unadjusted Model | Adjusted Modelb | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Serum LL-37 level by quartile | ||||
| Lowest (<34 ng/mL) | 0.50 (.32–.77) | .002c | 0.51 (.32–.80) | .003c |
| Second lowest (34–45.9 ng/mL) | 0.45 (.29–.69) | <.001c | 0.45 (.29–.71) | <.001c |
| Second highest (46–59.9 ng/mL) | 0.62 (.41–.92) | .02c | 0.64 (.43–.97) | .03c |
| Highest (≥60 ng/mL) | Reference | … | Reference | … |
| Covariates | ||||
| Age (mo) | ||||
| <2 mo | … | … | Reference | … |
| 2–5.9 mo | … | … | 0.97 (.65–1.46) | .90 |
| 6–12 mo | … | … | 0.9 (.55–1.48) | .67 |
| Female (vs male) sex | … | … | 1.51 (1.08–2.11) | .01c |
| Race/ethnicity | ||||
| Non-Hispanic white | … | … | Reference | … |
| Non-Hispanic black | … | … | 1.22 (.81–1.84) | .34 |
| Hispanic | … | … | 1.13 (.77–1.66) | .52 |
| Other | … | … | 1.17 (.48–2.86) | .74 |
| Prematurity (32–37 wk) | … | … | 1.33 (.90–1.97) | .16 |
| Breathing problems before index hospitalization | … | … | 1.57 (1.06–2.34) | .03c |
| Ever attended daycare | … | … | 1.36 (.92–1.99) | .12 |
| Sibling at home | … | … | 1.19 (.79–1.79) | .40 |
| Smoke exposure at home | … | … | 0.52 (.32–.86) | .01c |
| Antibiotic use before index hospitalization | … | … | 0.93 (.64–1.35) | .71 |
| Corticosteroid use before index hospitalization | … | … | 1.25 (.80–1.96) | .33 |
| Nasopharyngeal microbiota profile | ||||
| Moraxella dominant | … | … | Reference | … |
| Haemophilus dominant | … | … | 1.04 (.66–1.65) | .86 |
| Streptococcus dominant | … | … | 0.38 (.24–.62) | <.001c |
| Mixed | … | … | 0.72 (.47–1.09) | .12 |
Abbreviations: CI, confidence interval; OR, odds ratio.
aInfection with rhinovirus only or with other virus(es).
bMixed-effects logistic regression models adjusting for sites as random effects.
cSignificant at P < .05.
DISCUSSION
In this multicenter cohort study of >1000 infants hospitalized with bronchiolitis we found that lower levels of serum LL-37 (ie, cathelicidin) during hospitalization were significantly associated with increased intensive care and longer hospital LOS, even after adjusting for multiple patient level factors. We also found that infants in the highest quartile of serum LL-37 were less likely to have RSV bronchiolitis but more likely to have RV bronchiolitis. These results corroborate and extend previous findings from a small multicenter study (n = 82) that first linked low levels of serum cathelicidin to more severe bronchiolitis and RSV [12].
LL-37 is a part of the innate immune response and has extensive antimicrobial and immunomodulatory effects [10, 30] that have been shown to influence the severity of pulmonary conditions and mortality in murine and human studies. In mice, cathelicidin-related antimicrobial peptide, improved lung bacterial clearance and survival in response to Klebsiella pneumoniae [31]. In humans, cross-sectional analyses found that higher LL-37 levels (median, 36 ng/mL; IQR, 28.8–45.9) were associated with improved forced expiratory volume in one second of expiration [32]. Moreover, in 112 adults hospitalized with community-acquired pneumonia, values of serum LL-37 in the lowest tertile (overall median, 69 ng/mL; range, 13–263 ng/mL) were associated with a higher 30-day mortality (P = .053) [33]. In a nested case-control study among individuals initiating chronic hemodialysis (n = 279), patients with serum cathelicidin levels in the lowest tertile had an increased rate of death due to infection over 1 year (adjusted OR, 3.7; 95% confidence interval, 1.2–11.2) [7].
Concordant with these results and previous findings in 82 children with bronchiolitis [12], the present data demonstrate an inverse association between serum levels of LL-37 and severity of illness. It is unclear from these data, however, whether the higher serum LL-37 levels preceded the infection, were in response to it, or were due to confounding by a third factor. Indeed, neither these data nor previous studies have established causality between the serum level of LL-37 and severity of illness. However, given the underlying biology of LL-37 and the preponderance of data, further study of LL-37 as a potential treatment for bronchiolitis requiring intensive care seems warranted.
The present data suggest that higher circulating levels of LL-37 may be particularly useful for children with RSV bronchiolitis. LL-37 has direct antiviral effects against RSV [8–10], but it has also been shown to reduce RV replication in cystic fibrosis cells [11]. Our results, however, show a contrast between the relation of the LL-37 level and the viral etiology. Specifically, infants with serum LL-37 levels in the highest quartile were less likely to have RSV but more likely to have RV when compared with the other 3 LL-37 quartiles. We have now found in 2 separate bronchiolitis studies that high levels of LL-37 are associated with a decreased likelihood of RSV [12]. The new finding that high LL-37 levels were associated with RV is intriguing. Children with RV bronchiolitis have been shown in 2 separate cohorts to have shorter LOS (ie, lower severity of illness) than children with RSV bronchiolitis [3, 34]. The present data show that higher LL-37 levels are associated with less severe bronchiolitis. Although interesting, the details of the observed relationship between higher LL-37 levels, RV bronchiolitis, and decreased severity of illness are beyond the scope of the current results.
Bronchiolitis, however, is not simply a viral illness. The viruses that infect infants do so in an airway colonized with bacteria [21, 35]. Indeed, we found that children hospitalized with RSV or RV bronchiolitis have reproducible and distinct airway microbiota [35], including the RSV-Streptococcus association seen in the present data. We have also found that the severity of bronchiolitis is related not only to clinical factors [36] but also to the composition of the airway microbiota [21]. Specifically, when compared with a Moraxella-dominant airway microbiota, infants with a Haemophilus-dominant airway microbiota have more severe bronchiolitis [21], especially infants with low serum LL-37 levels [13]. Taken together, the present LL-37 results and previous data about viral-bacterial interactions suggest a complex interplay between the virus, the airway microbiota, and the host response. Ongoing multi-omic research and systems biology approaches will be needed to address these complex relationships.
Despite the complexity of the pathobiology of bronchiolitis, the association between vitamin D and cathelicidin provides one potential direction for treatment. In vitro 1,25-dihydroxyvitamin D increases the transcription of cathelicidin [37] and in vivo, vitamin D administration induces cathelicidin [27, 28]. In the present data and similar to previously reported findings [12], neither 25(OH)D nor bioavailable 25(OH)D was related to the quartile of serum LL-37. However, Quraishi and colleagues [27] demonstrated in a randomized placebo-controlled trial in 30 patients with sepsis that supplementation with high-dose cholecalciferol increased circulating levels of 25(OH)D, bioavailable 25(OH)D, and cathelicidin. However, they found a positive correlation only between bioavailable 25(OH)D and cathelicidin (Spearman ρ = 0.44; P = .03) [27]. Given the lack of beneficial pharmacotherapies for infants with bronchiolitis [38], these data support ongoing studies examining the induction of LL-37 using vitamin D [27]. In a separate, but related line of inquiry, these data also support efforts to create a synthetic, administrable version of cathelicidin [39].
The current study has several potential limitations. First, the study population was hospitalized infants, and the results may not be generalizable to infants in the outpatient setting. However, the results are relevant for the approximately 130000 infants who are hospitalized with bronchiolitis every year [2]. Second, the LL-37 levels were measured during the hospitalization, and prospective data are needed to understand the dynamics of LL-37 before the symptoms of bronchiolitis develop or early in the course of the illness. However, because only 3% of children with bronchiolitis require hospitalization [2], obtaining blood from infants before hospitalization would be technically and ethically challenging. Nonetheless, the present results suggest that further study of LL-37 in bronchiolitis is warranted. Third, it was beyond the scope of the present study to determine the specific cellular source (ie, neutrophils, epithelial cells, or macrophages) of the circulating LL-37. Finally, the timing of the increase in LL-37 and the LL-37 level required to potentially improve outcomes for infants with bronchiolitis remains uncertain. Finally, this observational study cannot prove causality between LL-37 levels and severity of illness or viral etiology. Nonetheless, the observed associations in the present data recapitulate previous findings [12] and remained significant after adjustment for multiple confounders and sites of care.
In this large, multicenter, ethnically diverse cohort of infants hospitalized with bronchiolitis, we found an inverse association between the level of LL-37 during hospitalization and the severity of illness, as measured by intensive care use and LOS. We also found an inverse relationship with RSV. Specifically, lower levels of LL-37 were associated with increased severity and RSV bronchiolitis. By contrast, higher levels of LL-37 were positively associated with RV. These findings support further investigation examining the role of LL-37 in bronchiolitis pathogenesis.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank the MARC-35 study hospitals and research personnel for their ongoing dedication to bronchiolitis and asthma research. We also thank Ashley F. Sullivan, MS, MPH for her unwavering commitment to the Emergency Medicine Network and MARC-35.
Disclaimer. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the grants U01 AI-087881, R01 AI-114552, R01 AI-108588, and R21 HL-129909 from the National Institute of Allergy and Infectious Diseases and UG3 OD-023253 from the Office of the Director at the National Institutes of Health.
Potential conflicts of interest. J. M. M. has provided bronchiolitis-related consultation for Regeneron. N. J. A. and J. F. P. own shares at Diversigen, a microbiome research company. P. A. P. provided bronchiolitis-related consultation for Gilead, Novavax, and Regeneron. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Yorita KL, Holman RC, Sejvar JJ, Steiner CA, Schonberger LB. Infectious disease hospitalizations among infants in the United States. Pediatrics 2008; 121:244–52. [DOI] [PubMed] [Google Scholar]
- 2. Hasegawa K, Tsugawa Y, Brown DF, Mansbach JM, Camargo CA Jr. Trends in bronchiolitis hospitalizations in the United States, 2000-2009. Pediatrics 2013; 132:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mansbach JM, Piedra PA, Teach SJ et al. ; MARC-30 Investigators Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch Pediatr Adolesc Med 2012; 166:700–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mansbach JM, McAdam AJ, Clark S et al. . Prospective multicenter study of the viral etiology of bronchiolitis in the emergency department. Acad Emerg Med 2008; 15:111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dürr UH, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta 2006; 1758:1408–25. [DOI] [PubMed] [Google Scholar]
- 6. Hilchie AL, Wuerth K, Hancock RE. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat Chem Biol 2013; 9:761–8. [DOI] [PubMed] [Google Scholar]
- 7. Gombart AF, Bhan I, Borregaard N et al. . Low plasma level of cathelicidin antimicrobial peptide (hCAP18) predicts increased infectious disease mortality in patients undergoing hemodialysis. Clin Infect Dis 2009; 48:418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Currie SM, Findlay EG, McHugh BJ et al. . The human cathelicidin LL-37 has antiviral activity against respiratory syncytial virus. PLoS One 2013; 8:e73659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Currie SM, Gwyer Findlay E, McFarlane AJ et al. . Cathelicidins have direct antiviral activity against respiratory syncytial virus in vitro and protective function in vivo in mice and humans. J Immunol 2016; 196:2699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harcourt JL, McDonald M, Svoboda P, Pohl J, Tatti K, Haynes LM. Human cathelicidin, LL-37, inhibits respiratory syncytial virus infection in polarized airway epithelial cells. BMC Res Notes 2016; 9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schögler A, Muster RJ, Kieninger E et al. . Vitamin D represses rhinovirus replication in cystic fibrosis cells by inducing LL-37. Eur Respir J 2016; 47:520–30. [DOI] [PubMed] [Google Scholar]
- 12. Mansbach JM, Piedra PA, Borregaard N et al. . Serum cathelicidin level is associated with viral etiology and severity of bronchiolitis. J Allergy Clin Immunol 2012; 130:1007–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hasegawa K, Mansbach JM, Ajami NJ et al. . Serum cathelicidin, nasopharyngeal microbiota, and disease severity among infants hospitalized with bronchiolitis. J Allergy Clin Immunol 2017; 139:1383–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Han JE, Jones JL, Tangpricha V et al. . High dose vitamin D administration in ventilated intensive care unit patients: a pilot double blind randomized controlled trial. J Clin Transl Endocrinol 2016; 4:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hasegawa K, Jartti T, Mansbach JM et al. . Respiratory syncytial virus genomic load and disease severity among children hospitalized with bronchiolitis: multicenter cohort studies in the United States and Finland. J Infect Dis 2015; 211:1550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leaf DE, Waikar SS, Wolf M, Cremers S, Bhan I, Stern L. Dysregulated mineral metabolism in patients with acute kidney injury and risk of adverse outcomes. Clin Endocrinol (Oxf) 2013; 79:491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Powe CE, Ricciardi C, Berg AH et al. . Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J Bone Miner Res 2011; 26:1609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beckham JD, Cadena A, Lin J et al. . Respiratory viral infections in patients with chronic, obstructive pulmonary disease. J Infect 2005; 50:322–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Knorr L, Fox JD, Tilley PA, Ahmed-Bentley J. Evaluation of real-time PCR for diagnosis of Bordetella pertussis infection. BMC Infect Dis 2006; 6:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Winchell JM, Thurman KA, Mitchell SL, Thacker WL, Fields BS. Evaluation of three real-time PCR assays for detection of Mycoplasma pneumoniae in an outbreak investigation. J Clin Microbiol 2008; 46:3116–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hasegawa K, Mansbach JM, Ajami NJ et al. . Association of nasopharyngeal microbiota profiles with bronchiolitis severity in infants hospitalized for bronchiolitis. Eur Respir J 2016; 48:1329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu GD, Chen J, Hoffmann C et al. . Linking long-term dietary patterns with gut microbial enterotypes. Science 2011; 334:105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rousseeuw PJ. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. J Comput Appl Math 1987; 20:53–65. [Google Scholar]
- 24. Jartti T, Hasegawa K, Mansbach JM, Piedra PA, Camargo CA Jr. Rhinovirus-induced bronchiolitis: lack of association between virus genomic load and short-term outcomes. J Allergy Clin Immunol 2015; 136:509–12.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mansbach JM, Piedra PA, Stevenson MD et al. ; MARC-30 Investigators Prospective multicenter study of children with bronchiolitis requiring mechanical ventilation. Pediatrics 2012; 130:e492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hasegawa K, Mansbach JM, Camargo CA Jr. Infectious pathogens and bronchiolitis outcomes. Expert Rev Anti Infect Ther 2014; 12:817–28. [DOI] [PubMed] [Google Scholar]
- 27. Quraishi SA, De Pascale G, Needleman JS et al. . Effect of cholecalciferol supplementation on vitamin D status and cathelicidin levels in sepsis: a randomized, placebo-controlled trial. Crit Care Med 2015; 43:1928–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J 2005; 19:1067–77. [DOI] [PubMed] [Google Scholar]
- 29. McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 2013; 8:e61217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andersson DI, Hughes D, Kubicek-Sutherland JZ. Mechanisms and consequences of bacterial resistance to antimicrobial peptides. Drug Resist Updat 2016; 26:43–57. [DOI] [PubMed] [Google Scholar]
- 31. Kovach MA, Ballinger MN, Newstead MW et al. . Cathelicidin-related antimicrobial peptide is required for effective lung mucosal immunity in gram-negative bacterial pneumonia. J Immunol 2012; 189:304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lambert AA, Kirk GD, Astemborski J et al. . A cross sectional analysis of the role of the antimicrobial peptide cathelicidin in lung function impairment within the ALIVE cohort. PLoS One 2014; 9:e95099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leow L, Simpson T, Cursons R, Karalus N, Hancox RJ. Vitamin D, innate immunity and outcomes in community acquired pneumonia. Respirology 2011; 16:611–6. [DOI] [PubMed] [Google Scholar]
- 34. Jartti T, Aakula M, Mansbach JM et al. . Hospital length-of-stay is associated with rhinovirus etiology of bronchiolitis. Pediatr Infect Dis J 2014; 33:829–34. [DOI] [PubMed] [Google Scholar]
- 35. Mansbach JM, Hasegawa K, Henke DM et al. . Respiratory syncytial virus and rhinovirus severe bronchiolitis are associated with distinct nasopharyngeal microbiota. J Allergy Clin Immunol 2016; 137:1909–1913.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mansbach JM, Piedra PA, Stevenson MD et al. ; MARC-30 Investigators Prospective multicenter study of children with bronchiolitis requiring mechanical ventilation. Pediatrics 2012; 130:e492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu PT, Stenger S, Li H et al. . Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006; 311:1770–3. [DOI] [PubMed] [Google Scholar]
- 38. Ralston SL, Lieberthal AS, Meissner HC et al. ; American Academy of Pediatrics Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014; 134:e1474–502. [DOI] [PubMed] [Google Scholar]
- 39. Hsieh IN, Hartshorn KL. The role of antimicrobial peptides in influenza virus infection and their potential as antiviral and immunomodulatory therapy. Pharmaceuticals (Basel) 2016; 9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.