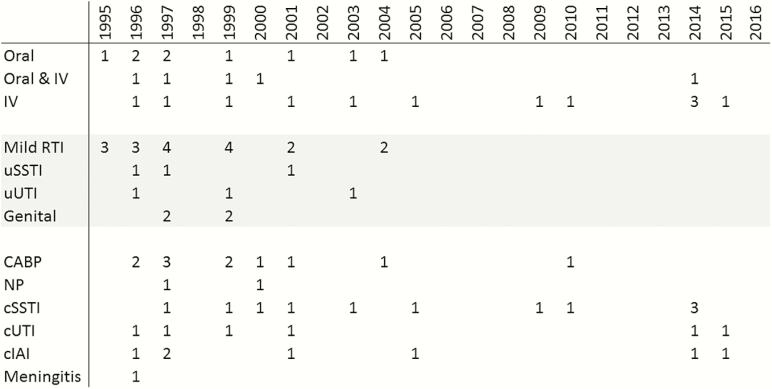
Figure 1.
Initial antibacterial approvals by route and indication, 1995–2016. Initial approvals of antibacterial agents from 1995 to 2016 were retrieved from CDERWatch and Drugs@FDA and are shown by approved route and number of initially approved indications (some agents were approved for >1 indication). As needed due to evolution of indication terminology, indications were grouped. The 2016 approval of bezlotoxumab to reduce recurrence of Clostridium difficile infection is not shown. Abbreviations: CABP, community-acquired bacterial pneumonia; cIAI, complicated intra-abdominal infection; cSSTI, complicated skin and skin structure infection; cUTI, complicated urinary tract infection; Genital, uncomplicated gonorrhea, prostatitis, nongonococcal urethritis, and chlamydia; IV, intravenous; Meningitis, bacterial meningitis; NP, nosocomial pneumonia including hospital- and ventilator-associated pneumonia; Mild RTI, acute otitis media, acute bacterial exacerbation of chronic bronchitis, acute sinusitis, and pharyngitis/tonsillitis; uSSTI, uncomplicated skin and skin structure infection; uUTI, uncomplicated urinary tract infection.