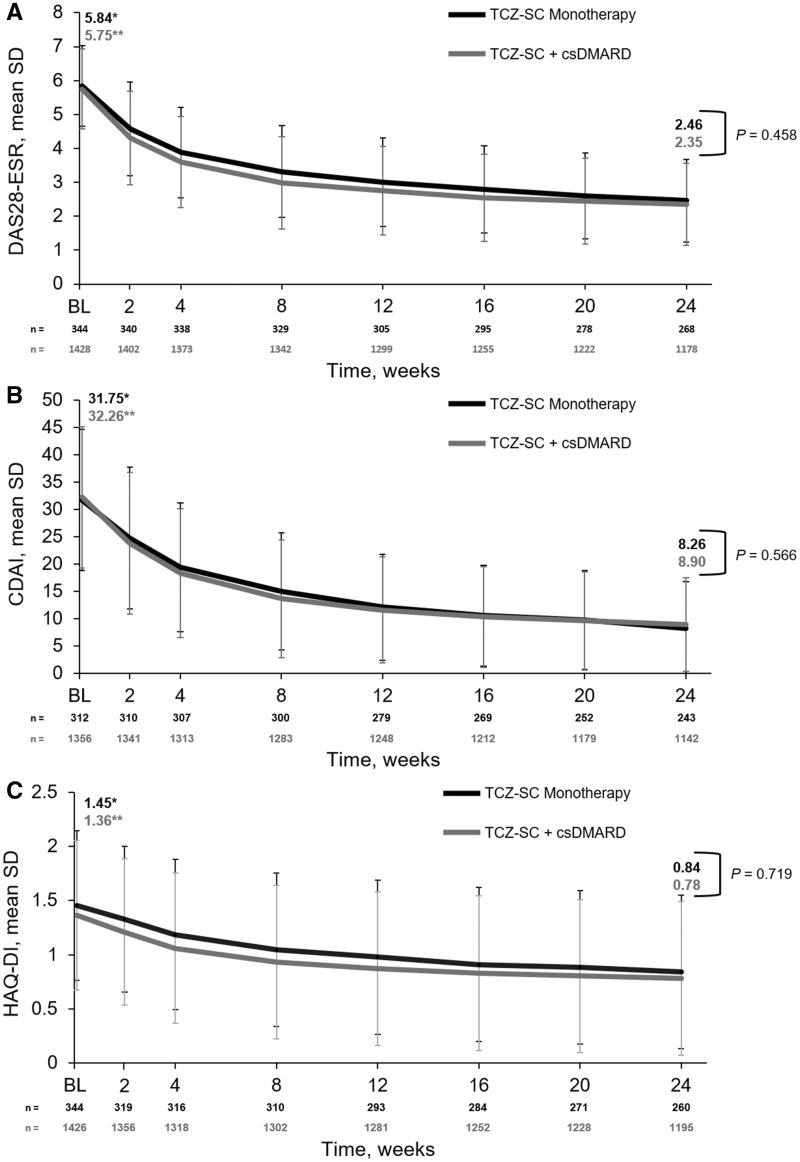
Fig. 3.
Mean (A) DAS28-ESR, (B) CDAI score and (C) HAQ-DI score over 24 weeks
*P < 0.0001 for TCZ-SC monotherapy comparing week 1 with week 24. **P < 0.0001 for TCZ-SC + csDMARD comparing week 1 with week 24. BL: baseline; CDAI: Clinical Disease Activity Index; csDMARD: conventional synthetic disease-modifying anti-rheumatic drug; DAS28-ESR: Disease Activity Score in 28 joints using erythrocyte sedimentation rate; HAQ-DI: Health Assessment Questionnaire-Disability Index; TCZ-SC: subcutaneous tocilizumab.