Abstract
STUDY QUESTION
Are body size across the life course and adult height associated with endometriosis?
SUMMARY ANSWER
Endometriosis is associated with lean body size during childhood, adolescence and adulthood; tall total adult height; and tall sitting height.
WHAT IS KNOWN ALREADY
The literature suggests that both adult body size and height are associated with endometriosis risk, but few studies have investigated the role of body size across the life course. Additionally, no study has investigated the relationships between components of height and endometriosis.
STUDY DESIGN, SIZE, DURATION
We used a nested case-control design within E3N (Etude Epidémiologique auprès de femmes de l'Education Nationale), a prospective cohort of French women. Data were updated every 2–3 years through self-administered questionnaires. Odds ratios (ORs) and 95% CIs were computed using logistic regression models adjusted for a priori confounding factors.
PARTICIPANTS/MATERIALS, SETTING, METHODS
A total of 2416 endometriosis cases were reported as surgically ascertained among the 61 208 included women.
MAIN RESULTS AND THE ROLE OF CHANCE
The odds of endometriosis were lower among women who reported having a large versus lean body size at 8 years (P for trend = 0.003), at menarche (P for trend < 0.0001) and at ages 20–25 years (P for trend < 0.0001). Women in the highest quartiles of height had statistically significantly increased odds of endometriosis compared to those in the lowest (<158 cm) (162–164 cm: OR = 1.28, 95% CI = 1.12–1.46; ≥165 cm: OR = 1.33, 95% CI = 1.18–1.49, P for trend < 0.0001). Statistically significantly increased odds were also observed among women with a taller sitting height (OR = 1.24, 95% CI = 1.05–1.47, P for trend = 0.01). Leg length was not statistically significantly associated with endometriosis.
LIMITATIONS REASONS FOR CAUTION
Endometriosis cases may be prone to misclassification; however, we restricted our case definition to surgically-confirmed cases, which showed a high validation rate. Body size is based on retrospective self-report, which may be subject to recall bias.
WIDER IMPLICATIONS OF THE FINDINGS
The results of this study suggest that endometriosis is positively associated with lean body size across the life course and total adult height. They also suggest that components of height are associated with endometriosis, which should be investigated further.
STUDY FUNDING/COMPETING INTEREST(S)
The Mutuelle Générale de l'Education Nationale (MGEN); the European Community; the French League against Cancer (LNCC); Gustave Roussy; the French Institute of Health and Medical Research (Inserm). L.V.F. was supported by a T32 grant (#HD060454) in reproductive, perinatal and pediatric epidemiology from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Cancer Institute (3R25CA057711) National Institutes of Health. M.K. was supported by a Marie Curie Fellowship within the seventh European Community Framework Programme (#PIOF-GA-2011–302078). The authors have no conflicts of interest to declare.
Keywords: anthropometry, body size, endometriosis, epidemiology, height, sitting height
Introduction
Endometriosis is a chronic gynecological disease characterized by the presence of endometrial tissue outside the uterine cavity (Giudice and Kao, 2004). It is related to infertility, chronic fatigue, chronic pelvic pain, dysmenorrhea (painful periods), dyspareunia (painful intercourse), dysuria (painful urination) and dyschezia (painful defecation) (Missmer and Cramer, 2003; Kennedy et al., 2005). Among women in the general population, endometriosis prevalence is ~6–10% (Missmer and Cramer, 2003; Missmer et al., 2004,b; Buck Louis et al., 2011). However, despite the high prevalence of endometriosis, considerable associated costs (Simoens et al., 2011), and substantial negative impacts on quality of life (Nnoaham et al., 2011), conclusive research on the etiology of endometriosis is limited.
Previous studies have suggested an inverse association between adult body weight and endometriosis risk (McCann et al., 1993; Parazzini et al., 1995; Signorello et al., 1997; Berube et al., 1998; Hemmings et al., 2004; Missmer et al., 2004b; Ferrero et al., 2005; Hediger et al., 2005; Buck Louis et al., 2007; Matalliotakis et al., 2008; Vitonis et al., 2010a; Lafay Pillet et al., 2012) and an increased risk of endometriosis with increasing adult height (Cramer et al., 1986; Signorello et al., 1997; Hediger et al., 2005). However, because endometriosis symptoms may present in early adolescence (Janssen et al., 2013), the current literature is unclear about whether these adult anthropometric characteristics are a cause or instead a consequence of endometriosis. The few studies that have investigated adolescent anthropometric features in relation to endometriosis have shown conflicting results (Hediger et al., 2005; Nagle et al., 2009; Vitonis et al., 2010a). Additionally, the association between height and endometriosis risk may be more complex than previously investigated. Indeed, height is a multi-factorial exposure, comprised of both sitting height and leg length. While the peak growth velocity is attained around menarche (Tanner et al., 1976), height components have been suggested to represent different hormonal and environmental exposures during childhood and adolescence (Gunnell et al., 2001; Rogol et al., 2002). The investigation of these factors in relation to endometriosis could thus potentially bring new information about the disease. However, no previous study has explored height components in relation to endometriosis risk.
This study aims to investigate the relationships between body size across the life course, adult height, components of adult height (sitting height and leg length) and odds of endometriosis in a large study of French women.
Methods
The E3N cohort
E3N (Etude Epidémiologique auprès de femmes de l'Education Nationale) is a prospective cohort study of 98 995 French women born between 1925 and 1950 and insured by a national health scheme primarily covering teachers (Clavel-Chapelon, 2015). Women were enrolled between 1989 and 1991 after returning a baseline self-administered questionnaire on their lifestyle and medical history along with an informed consent. Follow-up questionnaires were sent every 2–3 years, thereafter, and collected information on demographic and lifestyle factors as well as medical events and diagnoses. The E3N cohort received ethical approval from the French National Commission for Computerized Data and Individual Freedom (Commission Nationale Informatique et Libertés, CNIL).
Data collection
Body size and height definition and ascertainment
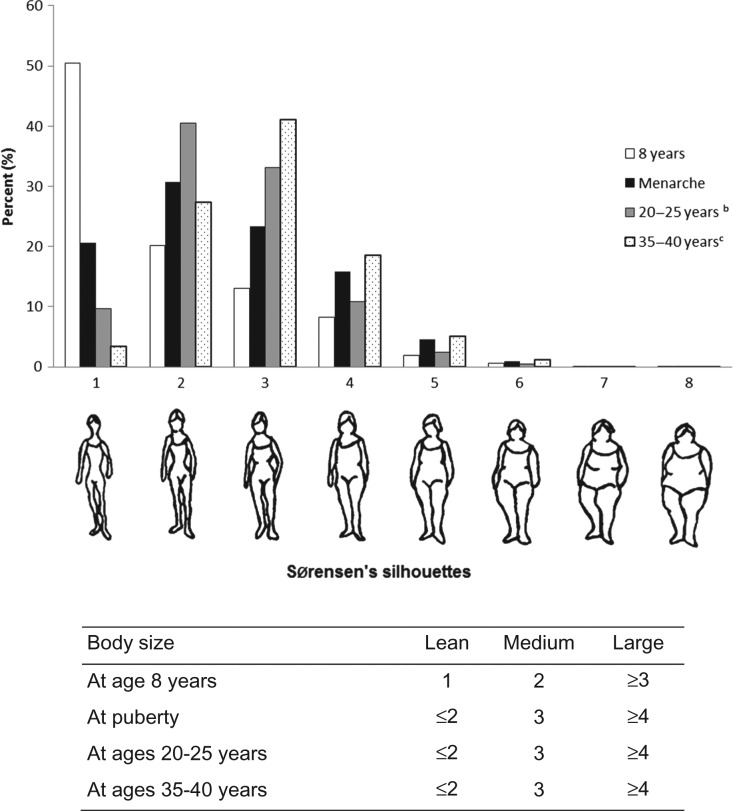
Body size from childhood through adulthood was assessed at inclusion via questionnaire using figure drawings (somatotypes) proposed by Sorensen et al., (1983) that ranged from 1 (leanest) to 8 (largest). Women were asked to self-report the drawing they perceived best reflected their body sizes at age 8 years, at time of menarche, 20–25 years and 35–40 years. For each of these variables, we created three categories (lean, medium and large) based on the underlying distribution of the data at each available period of life (Fig. 1).
Figure 1.
Distribution of Sørensen's pictograms selected by study participantsa to represent their body size at different ages, E3N cohort (n = 61 208). aTotals do not add-up because of missing exposure data: there were 3431 (5.6%) missing values for body size at age 8 years; 2453 (4.0%) for body size at time of menarche; 1802 (3.0%) for body size at 20–25 years and 1860 (3.1%) for body size at 35–40 years. bWomen who reported endometriosis before age 20 years (n = 28) were excluded in this analysis (the population was then constituted of n = 61 180 participants). cWomen who reported endometriosis before age 35 years (n = 657) were excluded in this analysis (the population was then constituted of n = 60 551 participants).
Adult height was self-reported at baseline and in 1995, 2000, and 2002 and averaged across questionnaires. In 1995, we collected self-reported sitting height by giving standardized instructions to women on how to self-measure. Women were instructed to sit upright on a hard seat with their buttocks and scapulas against a wall. While in that position, women used their own tape measure and an angle bracket at their head to measure sitting height. Women also reported seat heights. This allowed for a calculation of sitting height (as standing height minus seat height) and leg length (as standing height minus sitting height). Women missing measurements of sitting height and leg length had similar distributions of demographic and lifestyle variables than women with non-missing data (data not shown). For this analysis, height and its components were analyzed in quartiles.
A validation study of these height and body size measurements was conducted in 2002 among 152 women from the Paris centre of the cohort who provided a blood sample (Tehard et al., 2002). Correlation coefficients between self-reported and technician-measured anthropometric factors were 0.89 for height, 0.56 for sitting height and 0.85 for body size somatotype. To assess face validity of sitting height, we compared measurements of sitting height and sitting height percentage (sitting height/height × 100) for women pooled from the NHANES I (1971–1974) and II (1976–1980) (Frisancho, 1990) to measurements within E3N. We found similar ranges of sitting height between the two populations. Average sitting height measurements were shorter in the E3N cohort, however, no difference was greater than 0.9 cm.
Endometriosis case definition and ascertainment
The 1992 questionnaire retrospectively asked participants whether they had ever been diagnosed with endometriosis. Additional information about age at diagnosis, type of treatment and procedures that enabled diagnosis was also collected. Subsequent follow-up questionnaires continued to collect this information prospectively. Because endometriosis occurs mostly in women of reproductive age, we considered both prevalent cases (i.e. diagnosed before the 1992 questionnaire, reported retrospectively) and incident cases (i.e. diagnosed after the 1992 questionnaire, reported prospectively) and we used a nested case-control design with cumulative incidence sampling for analysis. Compared with prevalent cases, incident cases were more likely to be young at inclusion, to be parous, and to have ever used hormonal treatments, while they had similar height, BMI at inclusion, body size at ages 20–25 years, age at menarche, and menstrual cycle length before age 17 years, as previously described (Kvaskoff et al., 2013). We conducted sensitivity analyses by stratifying the results according to the prevalent or incident case status.
Because laparoscopic surgery is the gold-standard for endometriosis diagnosis (Giudice and Kao, 2004), we restricted our analyses to only those cases who reported endometriosis as diagnosed or treated by laparoscopy or surgery. We performed a validation study by sending a specific questionnaire to 200 randomly selected women who self-reported surgical treatment or diagnosis of endometriosis. We asked the women to confirm their date of diagnosis and to provide pathology or hospitalization reports, and the contact details of their physicians. A validation committee reviewed all documents; a mention of the presence of endometriosis was sought, and the physicians of the women were contacted in case of dubious reports, until a definitive conclusion was made. Among the 183 women who replied (92%), 75% (137 of 183) were confirmed, and the date of diagnosis was correctly reported in 82% of the validated cases (112 of 137). The self-reported diagnosis was incorrect in 17% of cases (31 of 183), and no clear conclusion could be drawn in 8% of cases (15 of 183) (i.e. no information on symptoms or disease history, or no medical report were provided). Among the 31 self-reports of endometriosis that were not confirmed, reasons included endometrial hyperplasia (54.9%), adenomyosis (29.0%) and abdominal pain of unknown cause (16.1%).
Assessment of covariates
Women reported their parity, age at menarche, breastfeeding status and duration for each live birth in the baseline and 1992 questionnaires, while data on menstrual cycle length during midlife was collected in 1992. Women reported physical activity at baseline, indicating their usual daily walking distance as well as weekly vigorous, moderate and cleaning activities as described in detail by Tehard et al., (2006). Beginning in 1992 and throughout follow-up, women reported current use of oral contraceptives (OCs) or premenopausal progestogens using a booklet containing color photographs of all hormonal medications in France at the time of the study to facilitate recall.
Population for analysis
Since endometriosis diagnosis in the older women of the cohort may represent mostly severe cases because of limited exposure to hormonal treatments and laparoscopy, we excluded women from the older birth cohort (1925–1935) from our study population (n = 16 857) in order to reduce this bias. We further excluded those with endometriosis who did not report treatment/diagnosis by surgery or laparoscopy (n = 1061). Since pelvic endometriosis is rare before menarche and after menopause (Missmer and Cramer, 2003), we excluded women who reported to have never menstruated (n = 26) and those who reported endometriosis diagnosis before menarche (n = 5) or after menopause (n = 518). We further excluded those with missing information on age at menarche (n = 1567) or on age at endometriosis diagnosis (n = 691). Finally, because a diagnosis of cancer may induce significant lifestyle and systemic changes that may in turn influence endometriosis risk, women with a history of cancer were excluded from the analyses (n = 19 209, including 671 cases of endometriosis). However, we verified that the results were not substantially modified in a sensitivity analysis re-including women with a cancer history (data not shown). Our final sample for analysis consisted of 61 208 women.
Statistical analyses
Statistical analyses were performed with SAS© version 9.3. Odds ratios (ORs) and 95% CIs were estimated using unconditional logistic regression models. Given the reported association between endometriosis and age (Missmer et al., 2004b; Kvaskoff et al., 2013), the associations between body size and height and endometriosis risk were first assessed in models adjusted for birth cohort (1935–1940, 1941–1945 and 1946–1950) and age at the last returned questionnaire (continuous). We additionally adjusted for the following covariates based on a priori hypothesized confounding relationships: parity (nulliparous, 1-2 children, ≥3 children) (Missmer et al., 2004a; Peterson et al., 2013), age at menarche (<12, 12–13, ≥14 years) (Missmer et al., 2004a; Kvaskoff et al., 2013), menstrual cycle length during midlife (irregular, or regular cycles of ≤24, 25–31 and ≥32 days) (Missmer et al., 2004a; Kvaskoff et al., 2013), physical activity at baseline (quartiles of MET-h/week) (Vitonis et al., 2010b), lifetime breastfeeding duration (no breastfeeding, <6 months, ≥6 months) (Missmer et al., 2004a), and smoking history (non, current, former smoker). Sensitivity analyses adjusted for ever use of OCs or premenopausal progestogens were additionally conducted to ensure that hormonal treatment for endometriosis did not confound our findings. We also checked adjustment for gravidity instead of parity, and in models assessing body size at different ages, a sensitivity model additionally adjusted for adult height.
In models of body size at ages 20–25 and 35–40, women who reported endometriosis diagnosis before the ages of 20 and 35 (n = 28 and n = 657, respectively) were excluded from the analyses to minimize reverse causation. In models of sitting height and leg length, analyses included women who returned the 1995 questionnaire only (n = 52 743). Tests for linear trend were performed in models where each factor of interest was entered as an ordinal variable. In additional analyses, we adjusted for body size at age 8 and at time of menarche to test if the effect of adult body size was independent of childhood body size. Splines of Ln(OR) of endometriosis odds and 95% CIs and components of height were estimated using three knots corresponding to quartile groups.
Because occurrence of endometriosis in our cohort was reported both retrospectively and prospectively, we repeated our analyses in prevalent and incident cases separately. We performed homogeneity tests to compare odd ratio estimates over strata (Hosmer and Lemeshow, 2000). A missing category was created for all variables of interest and numbers are provided as footnotes in the tables. For all other adjustment factors, missing values were imputed to the median or the modal category if occurring in <5% of observations; otherwise a missing category was created.
Results
A total of 2684 endometriosis cases were reported among the 61 208 included women. The average age of endometriosis diagnosis was 40.0 years (SD = 8.0 years). Compared to women without endometriosis, women with endometriosis were more likely to have an earlier age at menarche (24.9% versus 21.5%, <12 years), shorter menstrual cycle duration (8.2% versus 7.0%, ≤24 days), to be nulliparous (13.3% versus 10.7%), and to have ever used OCs (72.6% versus 69.3%) or premenopausal progestogens (66.0% versus 44.0%) (Table I).
Table I.
Characteristics of study participants, E3N cohort restricted to women born between 1935 and 1950 (n = 61 208).
| History of endometriosis | ||||
|---|---|---|---|---|
| Yes (n = 2416) | No (n = 58 792) | |||
| n | % | n | % | |
| Year of birth | ||||
| 1935–1940 | 502 | 20.8 | 16 472 | 28.0 |
| 1941–1945 | 788 | 32.6 | 18 086 | 30.8 |
| 1946–1950 | 1126 | 46.6 | 24 234 | 41.2 |
| Age at menarche | ||||
| <12 years | 600 | 24.9 | 12 623 | 21.5 |
| 12–13 years | 1203 | 49.8 | 30 186 | 51.3 |
| ≥14 years | 613 | 25.4 | 15 983 | 27.2 |
| Menstrual cycle length during midlife | ||||
| ≤24 days | 197 | 8.2 | 4115 | 7.0 |
| 25–31 days | 1933 | 80.0 | 48 012 | 81.7 |
| ≥32 days | 164 | 6.8 | 3704 | 6.3 |
| Irregular cycles | 122 | 5.0 | 2961 | 5.0 |
| Parity | ||||
| Nulliparous | 322 | 13.3 | 6277 | 10.7 |
| 1–2 live births | 1516 | 62.8 | 36 413 | 61.9 |
| ≥3 live births | 578 | 23.9 | 16 102 | 27.4 |
| Physical activity at baseline (MET-h/week) | ||||
| 0–25.99 | 618 | 25.6 | 15 172 | 25.8 |
| 26.00–37.16 | 601 | 24.9 | 14 697 | 25.0 |
| 37.17–52.8 | 662 | 27.4 | 15 288 | 26.0 |
| ≥52.9 | 535 | 22.1 | 13 635 | 23.2 |
| Lifetime breastfeeding | ||||
| None | 993 | 41.1 | 20 214 | 34.4 |
| ≤6 months | 981 | 40.6 | 22 278 | 37.9 |
| >6 months | 292 | 12.1 | 8088 | 13.8 |
| Unknown duration | 23 | 1.0 | 474 | 0.8 |
| Missing | 127 | 5.3 | 7738 | 13.2 |
| Use of OCs | ||||
| Ever | 1754 | 72.6 | 40 771 | 69.3 |
| Never | 662 | 27.4 | 18 021 | 30.7 |
| Use of premenopausal progestogens | ||||
| Ever | 1595 | 66.0 | 25 889 | 44.0 |
| Never | 821 | 34.0 | 32 903 | 56.0 |
| Skin tone | ||||
| Light (albino, very fair, fair and medium) | 2377 | 98.4 | 57 873 | 98.4 |
| Dark (brown and black) | 39 | 1.6 | 919 | 1.6 |
| Mean (SD) | Interquartile range | Mean (SD) | Interquartile range | |
| Adult height (cm)a | 162.6 (5.6) | 159.0–166.8 | 161.9 (5.6) | 158.0–166.0 |
| Sitting height (cm)b | 88.7 (13.0) | 83.0–89.0 | 88.5 (13.0) | 83.0–88.0 |
| Leg length (cm)b | 77.0 (5.0) | 74.0–80.0 | 76.8 (4.9) | 74.0–80.0 |
OCs, oral contraceptives.
aThere were 45 (0.1%) missing values for height.
bThere were 10 586 (13.9%) missing trunk length and leg length.
Compared to women who reported having a lean body size at age 8, those with a large estimated childhood body size had statistically significantly reduced odds of endometriosis later in life (OR: 0.86 (95% CI: 0.77–0.95)) (Table II). Women with reported medium and large body sizes at time of menarche had reduced odds of endometriosis compared to women with lean body size (medium: OR: 0.84 (0.76–0.94); large: OR: 0.79 (0.71–0.88)). We observed statistically significantly inverse dose-response relationships between both body size at age 8 and body size at time of menarche and endometriosis (P-values for trend, age 8:0.003, time of menarche: <0.0001). The relationships between childhood and adolescent body sizes and endometriosis were consistent across all levels of confounding adjustment.
Table II.
ORs and 95% CIs for endometriosis risk in relation to body size, E3N cohort restricted to women born between 1935 and 1950 (n = 61 208).
| na | Cases (n = 2416) | Age-adjusted ORb (95% CI) | Adjusted ORc (95% CI) | |
|---|---|---|---|---|
| Body size at age 8 years | ||||
| Lean | 30 855 | 1265 | 1.00 | 1.00 |
| Medium | 12 325 | 487 | 0.94 (0.85–1.05) | 0.93 (0.83–1.03) |
| Large | 14 597 | 540 | 0.88 (0.80–0.98) | 0.86 (0.77–0.95) |
| Ptrend | 0.02 | 0.003 | ||
| Body size at menarche | ||||
| Lean | 31 365 | 1338 | 1.00 | 1.00 |
| Medium | 14 267 | 529 | 0.86 (0.77–0.95) | 0.84 (0.76–0.94) |
| Large | 13 123 | 468 | 0.82 (0.73–0.91) | 0.79 (0.71–0.88) |
| Ptrend | <0.0001 | <0.0001 | ||
| Body size at ages 20–25 yearsd | ||||
| Lean | 30 682 | 1286 | 1.00 | 1.00 |
| Medium | 20 212 | 769 | 0.90 (0.82–0.98) | 0.89 (0.81–0.97) |
| Large | 8484 | 274 | 0.76 (0.67–0.87) | 0.73 (0.64–0.84) |
| Ptrend | <0.0001 | <0.0001 | ||
| Body size at ages 35–40 yearse | ||||
| Lean | 18 644 | 558 | 1.00 | 1.00 |
| Medium | 24 910 | 730 | 0.97 (0.87–1.09) | 0.96 (0.86–1.08) |
| Large | 15 137 | 430 | 0.95 (0.84–1.08) | 0.92 (0.81–1.05) |
| Ptrend | 0.47 | 0.20 | ||
OR, odds ratio.
aTotals do not add-up because of missing exposure data: there were 3431 (5.6%) missing values for body size at age 8 years; 2453 (4.0%) for body size at time of menarche; 1802 (3.0%) for body size at 20–25 years; 1860 (3.1%) for body size at 35–40 years (see also notes d and e).
bAdjusted for birth cohort and age at the last returned questionnaire.
cAdditionally adjusted for parity, age at menarche, menstrual cycle length during midlife, physical activity at baseline, lifetime breastfeeding and smoking habits.
dWomen who reported endometriosis before age 20 years (n = 28) were excluded in this analysis (the population was then constituted of n = 2388 cases and n = 58 792 non-cases).
eWomen who reported endometriosis before age 35 years (n = 657) were excluded in this analysis (the population was then constituted of n = 1759 cases and n = 58 792 non-cases).
We also found statistically significant inverse relationships between adult body size and endometriosis (Table II). Women with medium and large body sizes at ages 20–25 years were at decreased odds of endometriosis compared to those with a lean body size (medium: OR: 0.89 (0.81–0.97); large: OR: 0.73 (0.64–0.84); P-value for trend <0.0001). Women with a large body size at ages 35–40 years were also at decreased odds of endometriosis; however, the relationship failed to meet the threshold of statistical significance (OR: 0.92 (0.81–1.05); P-value for trend: 0.20). Again, these relationships were consistent across all levels of confounding adjustment, including after adjustment for body size at age 8 and body size at menarche.
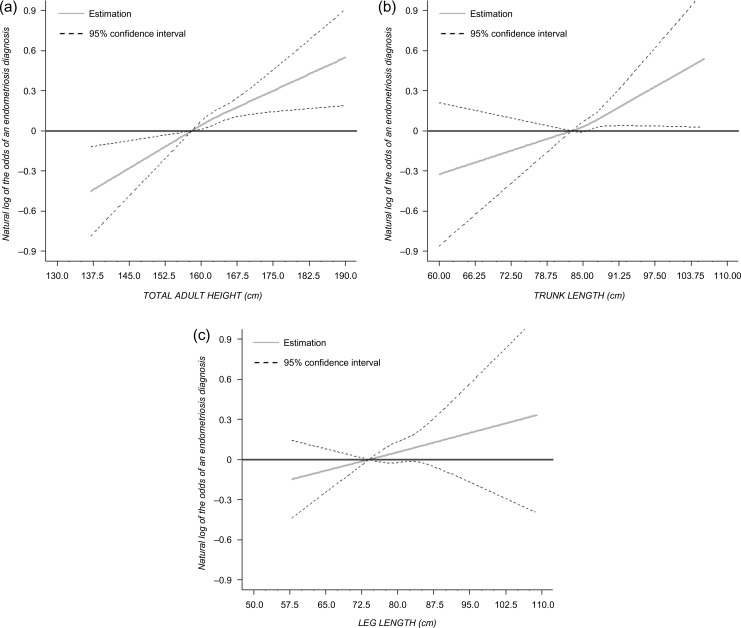
Height by quartiles was positively associated with the odds of endometriosis diagnosis (162–164 cm: OR: 1.28 (1.12–1.46); ≥165 cm: OR: 1.33 (1.18–1.49) compared with <158 cm) (linear trend P-value <0.0001) (Table III and Fig. 2A). When height was decomposed into its components, we observed a statistically significantly positive linear relationship between sitting height and endometriosis (P-value for trend: 0.01) (Fig. 2B). Women with a taller sitting height (≥87 cm) had increased odds of endometriosis compared to those with shorter sitting height (<82 cm) (OR: 1.24 (1.05–1.47)) (Table III). This relationship was attenuated slightly after adjustment for premenopausal progestogens. However, we found no statistically significant association between leg length and endometriosis (Table III and Fig. 2C).
Table III.
ORs and 95% CIs for endometriosis risk in relation to height and its components, E3N cohort restricted to subjects born between 1935 and 1950 (n = 61 208).
| na | Cases (n = 2416) | Age-adjusted ORb (95% CI) | Adjusted ORc(95% CI) | |
|---|---|---|---|---|
| Adult height (cm) | ||||
| <158 | 13 077 | 437 | 1.00 | 1.00 |
| 158–161 | 16 181 | 597 | 1.09 (0.96–1.24) | 1.11 (0.98–1.26) |
| 162–164 | 11 340 | 480 | 1.25 (1.10–1.43) | 1.28 (1.12–1.46) |
| ≥165 | 20 581 | 902 | 1.29 (1.15–1.45) | 1.33 (1.18–1.49) |
| Ptrend | <0.0001 | <0.0001 | ||
| Sitting height (cm) | ||||
| <82 | 5224 | 195 | 1.00 | 1.00 |
| 82–84 | 8566 | 358 | 1.11 (0.93–1.33) | 1.12 (0.94–1.34) |
| 85–86 | 7819 | 323 | 1.09 (0.91–1.30) | 1.10 (0.91–1.32) |
| ≥87 | 12 254 | 574 | 1.22 (1.03–1.44) | 1.24 (1.05–1.47) |
| Ptrend | 0.02 | 0.01 | ||
| Leg length (cm) | ||||
| <74 | 8238 | 347 | 1.00 | 1.00 |
| 74–76 | 8404 | 335 | 0.94 (0.81–1.09) | 0.95 (0.81–1.11) |
| 77–79 | 8014 | 352 | 1.04 (0.89–1.21) | 1.06 (0.91–1.23) |
| ≥79 | 9207 | 416 | 1.07 (0.92–1.24) | 1.10 (0.95–1.27) |
| Ptrend | 0.20 | 0.10 | ||
aTotals do not add-up because of missing exposure data: there were 29 (0.1%) missing values for height and 8465 (20.0%) for sitting height and leg length. Categories based on distribution of data (quartiles). For sitting height and leg length, analyses were restricted to women who responded to the 1995 questionnaire and with available value of sitting height and leg length (n = 42 328).
bAdjusted for birth cohort and age at the last returned questionnaire.
cAdditionally adjusted for parity, age at menarche, menstrual cycle length during midlife, physical activity at baseline, lifetime breastfeeding and smoking habits.
Figure 2.
Association between endometriosis and height measures using Restricted Cubic Splines with three knots: (a) standing height reference 158 cm; (b) trunk length reference 83 cm and (c) leg length reference 74 cm.
Stratification analyses were conducted according to the incident (n = 577) or prevalent (n = 1839) status of endometriosis cases. Relationships between body size at age 8 years, body size at menarche, body size at ages 35–40 years, height, sitting height and leg length were not statistically significantly different between groups (P-values for heterogeneity: 0.13, 0.12, 0.20, 0.70, 0.30 and 0.92, respectively.) The relationship between body size at ages 20–25 years and endometriosis was stronger among prevalent endometriosis cases (P-value for trend: <0.0001) compared to incident cases (P-value for trend: 0.79) (P-value for heterogeneity: 0.01).
Discussion
Within this large French cohort, we found that body size throughout the life course and adult height were associated with endometriosis. Women with a large body size, whether as a child or as an adult, had decreased odds of endometriosis diagnosis later in life, while women with a tall height (i.e. ≥165 cm) had increased odds of the disease. Our study also showed an association between components of height and endometriosis, which had never been investigated.
Our analysis found that body sizes at age 8 and at age of menarche were statistically significantly and inversely associated with odds of endometriosis. These findings are consistent with the results from the three previous studies that have investigated childhood and adolescent body size and BMI in relation to risk of endometriosis. In the Nurses’ Health Study II (NHSII), a prospective cohort of over 116 000 US nurses, body size in childhood (i.e. at age 5, age 10 and averaged between the ages of 5 and 10 years) was inversely associated with endometriosis risk (Vitonis et al., 2010a). This finding was supported by a US case-control study that used recalled body size in 5-year increments from ages 15 to 45 and found that cases reported smaller body sizes than controls (Hediger et al., 2005). However, an Australian case-control study reported a positive linear relationship between self-reported overweight at age 10 and risk of endometriosis, but also a relationship between mother-reported underweight at age 16 and endometriosis risk (Nagle et al., 2009).
Body size in childhood results from multiple exposures and likely reflects genetic make-up, the early life environment, nutritional status, exposure to infections and circulating hormonal status during childhood (Maes et al., 1997; Rogol et al., 2002; Wardle et al., 2008). Childhood body size influences age at menarche and at thelarche, which also may influence body size later in life (Berkey et al., 2000). While a large childhood body size may cause earlier timing of menarche for some girls, for others it may indicate high androgenic levels and features of polycystic ovarian syndrome, which may lead to irregular and long menstrual cycles (Goodarzi et al., 2011). Despite the consistent relationship between endometriosis and early age at menarche, including in this cohort (Kvaskoff et al., 2013), the relationship between body size and endometriosis appears to be acting independently of this association, since the relationship persisted after statistical adjustment in ours and other studies.
The literature has consistently suggested an inverse relationship between adult body size and endometriosis (Cramer et al., 1986; McCann et al., 1993; Signorello et al., 1997; Missmer et al., 2004b; Ferrero et al., 2005; Hediger et al., 2005; Nagle et al., 2009; Lafay Pillet et al., 2012; Shah et al., 2013), in accordance with our findings. In our study, while body sizes at ages 20–25 and 35–40 were both inversely associated with endometriosis, only body size at ages 20–25 reached the threshold of statistical significance. Sensitivity analyses showed that the relationship with body size at ages 20–25 was stronger among prevalent cases at cohort enrollment, which may partially be driven by limited power among women diagnosed at older ages. Our finding that early adult body size (at ages 20–25) is a stronger predictor of endometriosis than body size at ages 35–40 is consistent with data from the NHSII, which found that the relationship between body size and endometriosis was primarily driven by early adult BMI, at age 18, as opposed to BMI immediately before diagnosis (Shah et al., 2013). Sensitivity analyses in our study also indicated that body size at ages 20–25 was related to odds of endometriosis independently of body size at age 8 and at time of menarche. Only one study explored these relations according to endometriosis staging and found that more severe Stages (III–IV) were associated with leaner adulthood body size compared to less severe cases (P-value for trend: 0.002) (Yi et al., 2009). Unfortunately, data on endometriosis staging were not available in the E3N cohort. Future research should investigate endometriosis staging heterogeneity to better understand these relations.
While recent genetic research has shown associations between endometriosis and a genetic locus associated with waist to-hip ratio, no associations were found with known genetic BMI variants (Rahmioglu et al., 2015). This may suggest that the association between BMI, body size and endometriosis may be operating through common environmental factors or biological mechanisms associated with the distribution of adipose tissue (e.g. hormone-related mechanisms) rather than overall adiposity (e.g. altered metabolism associated with high BMI).
Regarding adult height, our findings are consistent with the previous research and suggest that women with taller adult height are at increased risk of endometriosis. Three case-control studies have reported an increased likelihood of endometriosis with taller height (Cramer et al., 1986; Signorello et al., 1997; Hediger et al., 2005). While no statistically significant linear association was found in the NHSII cohort study, taller women were at statistically significant increased risk of endometriosis compared to the shorter referent category of women (Missmer et al., 2004b; Shah et al., 2013).
Like body size, attained adult height is influenced by various factors prior to menarche, including time of menarche and growth velocity, which in turn may be influenced by early life and in utero nutritional status, environment, and genetics (Gunnell et al., 2001). Adult height has been consistently linked with an increased risk of chronic diseases such as cardiovascular diseases and several cancer types, including ovarian and breast cancers (Davey Smith et al., 2000; Gunnell et al., 2001; Schouten et al., 2008; Green et al., 2011). Height-associated loci are associated with neoplastic growth (Tripaldi et al., 2013), which may help elucidate the association between height and cancer and the associations found between endometriosis and cancer risk (Kvaskoff et al., 2014,2015; Farland et al., 2016).
In women, final adult height is strongly linked with timing of menarche, because surges of sex hormones at menarche result in the fusion of the epiphyseal growth plates, which limits future growth (Eastell, 2005). Because height may represent a variety of early life exposures, the association between height and endometriosis is unclear. However, understanding associations with components of height (sitting height and leg length) may provide new insights into timing of growth that is an important exposure window for endometriosis.
Our analysis found a statistically significant association between sitting height and odds of endometriosis. Leg length, which has most consistently been positively associated with cancer risk, is thought to reflect growth prior to puberty (Gunnell et al., 2001) and has been suggested as a marker for nutritional improvements on the population level (Leitch, 1951; Fredriks et al., 2005), whereas sitting height may reflect a stronger pubertal growth spurt (Schooling et al., 2007) and altered exposure to growth hormone and insulin-like growth factor-I (IGF-I) surges during peak growth (Rogol et al., 2002). Taller sitting height may also reflect a longer period of post-menarcheal growth. While some studies have linked endometriosis with higher IGF-I levels, the findings have been inconsistent (Giudice et al., 1994; Gurgan et al., 1999; Kim et al., 2011; Mu et al., 2015). Thus, our findings may suggest that potentially high hormonal exposures at the time of puberty may influence endometriosis risk, while nutrition and infectious exposures in childhood, as reflected by adult leg length, may not have a strong influence on adult endometriosis risk.
This study has several strengths, including its large sample size, detailed information on endometriosis diagnosis, and detailed available data on anthropometric features and endometriosis risk factors. However, some limitations should be considered in the interpretation of the findings. Endometriosis diagnosis was based on self-report, which could have induced misclassification; however, we restricted our endometriosis case definition to those cases treated or diagnosed by surgery or laparoscopy, which should have substantially decreased misclassification given the high validation rate in our validation study. Because endometriosis can be asymptomatic, in the absence of a surgical assessment, some endometriosis cases may have been misclassified in the non-case group. However, the impact of false negatives in such a large population of non-cases is likely to be low and would mostly result in diluting associations. Diagnosis through laparoscopy could also reflect selection of severe cases of endometriosis, although previous reports do not support this hypothesis (Missmer et al., 2004b).
Case status and covariate status were reported retrospectively and are thus subject to recall bias and reverse causation. Although reproducibility could not be tested for all covariates in our analysis, previous validation studies on various self-reported factors in the E3N cohort showed high agreement levels (Clavel-Chapelon and Dormoy-Mortier, 1998; Tehard et al., 2002; Kvaskoff et al., 2009), suggesting a high quality of the cohort data. We found lower correlation between self-reported and measured sitting height than for other studied anthropometric parameters, thus our results on sitting height should be interpreted cautiously; however, no absolute difference between the two measurements exceeded 1.1 cm, so that categorization in quartiles made the risk of misclassification bias unlikely (Tehard et al., 2002; Fagherazzi et al., 2012). In addition, misclassification of sitting height would most likely be non-differential; therefore, potential misclassification would result in an underestimation of the association between sitting height and endometriosis. Our population was restricted to women born in 1935–1950, which reduced the selection of severe cases in older women who had limited exposure to hormonal treatments and laparoscopy, as well as measurement error of adult height due to shrinkage. However, we expect any misclassification of adult height due to shrinkage to be non-differential with respect to endometriosis diagnosis. Ideally, future studies should confirm these findings using sitting height as measured by a technician. In addition, it has been suggested that measures of sitting height and leg length may be misclassified due to gluteal size in populations with high percentage of overweight and obese women (Bogin and Varela-Silva, 2008); however, we expect this bias to be minimal in our population given its very low level of obesity, 3% at time of anthropometric measurement.
There have been reported racial/ethnic differences in adult body size (Frisancho, 1990) and risk of endometriosis (Missmer et al., 2004b). However, questions regarding racial/ethnic origin were not collected in this cohort and thus possible differences in the relationship between height or body size, and risk of endometriosis across racial/ethnic groups could not be adequately assessed, which could be a limitation to the interpretation of the results.
Additionally, as in all observational studies, we cannot completely rule out possible residual confounding due to unmeasured factors; however, this bias is likely minimal given the minimal confounding observed after adjustment by a priori confounding factors.
OCs became available in France during 1960s; thus, older members of our cohort were less likely to be exposed to hormonal treatments. However, we found no effect modification by birth cohort and the confounding by these treatments was minimal in all models. Therefore, any differences in timing of exposure to OCs and progestogens should have little impact on our findings.
Finally, women from this cohort were all insured by a national health scheme mostly covering teachers and co-workers (the Mutuelle Générale de L'Education Nationale). Therefore, the results of this work may not be generalizable to populations with different education or socio-economic backgrounds, or with different height and weight distributions. However, we believe that the general etiologic findings that we observed between height, body size and endometriosis should be consistent across populations.
In sum, lean body size across the life course, height and sitting height were associated with increased odds of endometriosis in this large French study. The direction and magnitude of the relationships between adolescent body size, overall adult height and endometriosis were consistent with the current literature and reinforce the importance of the early life environment in relation to endometriosis. Our research suggests for the first time that components of height are associated with endometriosis. This finding may provide novel insights into the etiology of endometriosis and its relationship with total height. Future research should focus on further identifying the hormonal and environmental factors that underlie the associations between anthropometric features and endometriosis risk, and understanding the most critical exposure window for these factors with regards to endometriosis etiology.
Acknowledgements
The authors are indebted to all participants for their continued participation, and to Dr Nicolas Chopin, Dr Hervé Foulot and Prof. Charles Chapron for reviewing endometriosis cases for the validation study. They are grateful to all members of the E3N-EPIC study group, particularly to Marie Fangon, Pascale Gerbouin-Rérolle, Lyan Hoang, Céline Kernaleguen, Camille Laplanche, Maryvonne Niravong and Maxime Valdenaire for their technical assistance.
Authors’ roles
M.K. conceived, designed and supervised the study. L.V.F. drafted the manuscript. A.B., G.G. and A.G. performed the statistical analysis. L.V.F., S.A.M., A.B., G.G., A.G., F.C.-C., S.M., M.C.B.-R. and M.K. critically reviewed the manuscript and approved its final version.
Funding
The E3N study was funded by the Mutuelle Générale de l'Education Nationale (MGEN); the European Community; the French League against Cancer (LNCC); Gustave Roussy; and the French Institute of Health and Medical Research (Inserm). L.V.F. was supported by a T32 grant (#HD060454) in reproductive, perinatal, and pediatric epidemiology from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Cancer Institute (3R25CA057711) National Institutes of Health.
Conflict of interest
None declared.
References
- Berkey CS, Gardner JD, Frazier AL, Colditz GA. Relation of childhood diet and body size to menarche and adolescent growth in girls. Am J Epidemiol 2000;152:446–452. [DOI] [PubMed] [Google Scholar]
- Berube S, Marcoux S, Maheux R. Characteristics related to the prevalence of minimal or mild endometriosis in infertile women. Canadian Collaborative Group on Endometriosis. Epidemiology 1998;9:504–510. [DOI] [PubMed] [Google Scholar]
- Bogin B, Varela-Silva MI. Fatness biases the use of estimated leg length as an epidemiological marker for adults in the NHANES III sample. Int J Epidemiol. 2008;37:201–209. doi:10.1093/ije/dym254. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Hediger ML, Pena JB. Intrauterine exposures and risk of endometriosis. Hum Reprod 2007;22:3232–3236. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Hediger ML, Peterson CM, Croughan M, Sundaram R, Stanford J, Chen Z, Fujimoto VY, Varner MW, Trumble A et al. . Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil Steril 2011;96:360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavel-Chapelon F. Cohort profile: the French E3N Cohort Study. Int J Epidemiol 2015;44:801–809. [DOI] [PubMed] [Google Scholar]
- Clavel-Chapelon F, Dormoy-Mortier N. A validation study on status and age of natural menopause reported in the E3N cohort. Maturitas 1998;29:99–103. [DOI] [PubMed] [Google Scholar]
- Cramer DW, Wilson E, Stillman RJ, Berger MJ, Belisle S, Schiff I, Albrecht B, Gibson M, Stadel BV, Schoenbaum SC. The relation of endometriosis to menstrual characteristics, smoking, and exercise. JAMA 1986;255:1904–1908. [PubMed] [Google Scholar]
- Davey Smith G, Hart C, Upton M, Hole D, Gillis C, Watt G, Hawthorne V. Height and risk of death among men and women: aetiological implications of associations with cardiorespiratory disease and cancer mortality. J Epidemiol Community Health 2000;54:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastell R. Role of oestrogen in the regulation of bone turnover at the menarche. J Endocrinol 2005;185:223–234. [DOI] [PubMed] [Google Scholar]
- Fagherazzi G, Vilier A, Boutron-Ruault MC, Clavel-Chapelon F, Mesrine S. Height, sitting height, and leg length in relation with breast cancer risk in the E3N cohort. Cancer Epidemiol Biomarkers Prev 2012;21:1171–1175. [DOI] [PubMed] [Google Scholar]
- Farland LV, Tamimi RM, Eliassen AH, Spiegelman D, Hankinson SE, Chen WY, Missmer SA. Laparoscopically confirmed endometriosis and breast cancer in the Nurses’ Health Study II. Obstet Gynecol 2016;128:1025–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero S, Anserini P, Remorgida V, Ragni N. Body mass index in endometriosis. Eur J Obstet Gynecol Reprod Biol 2005;121:94–98. [DOI] [PubMed] [Google Scholar]
- Fredriks AM, van Buuren S, van Heel WJ, Dijkman-Neerincx RH, Verloove-Vanhorick SP, Wit JM. Nationwide age references for sitting height, leg length, and sitting height/height ratio, and their diagnostic value for disproportionate growth disorders. Arch Dis Child 2005;90:807–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisancho AR. Anthropometric Standards for the Asessment of Growth and Nutrional Status. Ann Arbor: The University of Michigan Press, 1990. [Google Scholar]
- Giudice LC, Dsupin BA, Gargosky SE, Rosenfeld RG, Irwin JC. The insulin-like growth factor system in human peritoneal fluid: its effects on endometrial stromal cells and its potential relevance to endometriosis. J Clin Endocrinol Metab 1994;79:1284–1293. [DOI] [PubMed] [Google Scholar]
- Giudice LC, Kao LC. Endometriosis. Lancet 2004;364:1789–1799. [DOI] [PubMed] [Google Scholar]
- Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol 2011;7:219–231. [DOI] [PubMed] [Google Scholar]
- Green J, Cairns BJ, Casabonne D, Wright FL, Reeves G, Beral V. Height and cancer incidence in the Million Women Study: prospective cohort, and meta-analysis of prospective studies of height and total cancer risk. Lancet Oncol 2011;12:785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnell D, Okasha M, Smith GD, Oliver SE, Sandhu J, Holly JM. Height, leg length, and cancer risk: a systematic review. Epidemiol Rev 2001;23:313–342. [DOI] [PubMed] [Google Scholar]
- Gurgan T, Bukulmez O, Yarali H, Tanir M, Akyildiz S. Serum and peritoneal fluid levels of IGF I and II and insulinlike growth binding protein-3 in endometriosis. J Reprod Med 1999;44:450–454. [PubMed] [Google Scholar]
- Hediger ML, Hartnett HJ, Louis GM. Association of endometriosis with body size and figure. Fertil Steril 2005;84:1366–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings R, Rivard M, Olive DL, Poliquin-Fleury J, Gagne D, Hugo P, Gosselin D. Evaluation of risk factors associated with endometriosis. Fertil Steril 2004;81:1513–1521. [DOI] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S. Applied Logistic Regression. New-York: John Wiley & Sons Inc, 2000. [Google Scholar]
- Janssen EB, Rijkers AC, Hoppenbrouwers K, Meuleman C, D'Hooghe TM. Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: a systematic review. Hum Reprod Update 2013;19:570–582. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Bergqvist A, Chapron C, D'Hooghe T, Dunselman G, Greb R, Hummelshoj L, Prentice A, Saridogan E. ESHRE guideline on the diagnosis and treatment of endometriosis. Hum Reprod 2005;20:2698–2704. [DOI] [PubMed] [Google Scholar]
- Kim H, Park JH, Ku SY, Kim SH, Choi YM, Kim JG. Association between endometriosis and polymorphisms in insulin-like growth factors (IGFs) and IGF-I receptor genes in Korean women. Eur J Obstet Gynecol Reprod Biol 2011;156:87–90. [DOI] [PubMed] [Google Scholar]
- Kvaskoff M, Bijon A, Clavel-Chapelon F, Mesrine S, Boutron-Ruault MC. Childhood and adolescent exposures and the risk of endometriosis. Epidemiology 2013;24:261–269. [DOI] [PubMed] [Google Scholar]
- Kvaskoff M, Han J, Qureshi AA, Missmer SA. Pigmentary traits, family history of melanoma and the risk of endometriosis: a cohort study of US women. Int J Epidemiol 2014;43:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvaskoff M, Mesrine S, Clavel-Chapelon F, Boutron-Ruault MC. Endometriosis risk in relation to naevi, freckles and skin sensitivity to sun exposure: the French E3N cohort. Int J Epidemiol 2009;38:1143–1153. [DOI] [PubMed] [Google Scholar]
- Kvaskoff M, Mu F, Terry KL, Harris HR, Poole EM, Farland L, Missmer SA. Endometriosis: a high-risk population for major chronic diseases. Hum Reprod Update 2015;21:500–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafay Pillet MC, Schneider A, Borghese B, Santulli P, Souza C, Streuli I, de Ziegler D, Chapron C. Deep infiltrating endometriosis is associated with markedly lower body mass index: a 476 case-control study. Hum Reprod 2012;27:265–272. [DOI] [PubMed] [Google Scholar]
- Leitch I. Growth and health. Br J Nutr 1951;5:142–151. [DOI] [PubMed] [Google Scholar]
- Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet 1997;27:325–351. [DOI] [PubMed] [Google Scholar]
- Matalliotakis IM, Cakmak H, Fragouli YG, Goumenou AG, Mahutte NG, Arici A. Epidemiological characteristics in women with and without endometriosis in the Yale series. Arch Gynecol Obstet 2008;277:389–393. [DOI] [PubMed] [Google Scholar]
- McCann SE, Freudenheim JL, Darrow SL, Batt RE, Zielezny MA. Endometriosis and body fat distribution. Obstet Gynecol 1993;82:545–549. [PubMed] [Google Scholar]
- Missmer SA, Cramer DW. The epidemiology of endometriosis. Obstet Gynecol Clin North Am 2003;30:1–19. [DOI] [PubMed] [Google Scholar]
- Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Malspeis S, Willett WC, Hunter DJ. Reproductive history and endometriosis among premenopausal women. Obstet Gynecol 2004. a;104:965–974. [DOI] [PubMed] [Google Scholar]
- Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol 2004. b;160:784–796. [DOI] [PubMed] [Google Scholar]
- Mu F, Hankinson SE, Schernhammer E, Pollak MN, Missmer SA. A prospective study of insulin-like growth factor 1, its binding protein 3, and risk of endometriosis. Am J Epidemiol 2015;182:148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle CM, Bell TA, Purdie DM, Treloar SA, Olsen CM, Grover S, Green AC. Relative weight at ages 10 and 16 years and risk of endometriosis: a case-control analysis. Hum Reprod 2009;24:1501–1506. [DOI] [PubMed] [Google Scholar]
- Nnoaham KE, Hummelshoj L, Webster P, d'Hooghe T, de Cicco Nardone F, de Cicco Nardone C, Jenkinson C, Kennedy SH, Zondervan KT; World Endometriosis Research Foundation Global Study of Women's Health consortium. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril 2011;96:366–373.e8. doi:10.1016/j.fertnstert.2011.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parazzini F, Ferraroni M, Fedele L, Bocciolone L, Rubessa S, Riccardi A. Pelvic endometriosis: reproductive and menstrual risk factors at different stages in Lombardy, northern Italy. J Epidemiol Community Health 1995;49:61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CM, Johnstone EB, Hammoud AO, Stanford JB, Varner MW, Kennedy A, Chen Z, Sun L, Fujimoto VY, Hediger ML et al. . Risk factors associated with endometriosis: importance of study population for characterizing disease in the ENDO Study. Am J Obstet Gynecol 2013;208:451 e451–451 e411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmioglu N, Macgregor S, Drong AW, Hedman AK, Harris HR, Randall JC, Prokopenko I, Nyholt DR, Morris AP, Montgomery GW et al. . Genome-wide enrichment analysis between endometriosis and obesity-related traits reveals novel susceptibility loci. Hum Mol Genet 2015;24:1185–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogol AD, Roemmich JN, Clark PA. Growth at puberty. J Adolesc Health 2002;31:192–200. [DOI] [PubMed] [Google Scholar]
- Schooling CM, Jiang C, Lam TH, Thomas GN, Heys M, Bmbs, Lao X, Zhang W, Adab P, Cheng KK et al. . Height, its components, and cardiovascular risk among older Chinese: a cross-sectional analysis of the Guangzhou Biobank Cohort Study. Am J Public Health 2007;97:1834–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten LJ, Rivera C, Hunter DJ, Spiegelman D, Adami HO, Arslan A, Beeson WL, van den Brandt PA, Buring JE, Folsom AR et al. . Height, body mass index, and ovarian cancer: a pooled analysis of 12 cohort studies. Cancer Epidemiol Biomarkers Prev 2008;17:902–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah DK, Correia KF, Vitonis AF, Missmer SA. Body size and endometriosis: results from 20 years of follow-up within the Nurses’ Health Study II prospective cohort. Hum Reprod 2013;28:1783–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorello LB, Harlow BL, Cramer DW, Spiegelman D, Hill JA. Epidemiologic determinants of endometriosis: a hospital-based case-control study. Ann Epidemiol 1997;7:267–741. [DOI] [PubMed] [Google Scholar]
- Simoens S, Hummelshoj L, Dunselman G, Brandes I, Dirksen C, D'Hooghe T. Endometriosis cost assessment (the EndoCost study): a cost-of-illness study protocol. Gynecol Obstet Invest 2011;71:170–176. [DOI] [PubMed] [Google Scholar]
- Sorensen TI, Stunkard AJ, Teasdale TW, Higgins MW. The accuracy of reports of weight: children's recall of their parents’ weights 15 years earlier. Int J Obes 1983;7:115–122. [PubMed] [Google Scholar]
- Tanner JM, Whitehouse RH, Marubini E, Resele LF. The adolescent growth spurt of boys and girls of the Harpenden growth study. Ann Hum Biol 1976;3:109–126. [DOI] [PubMed] [Google Scholar]
- Tehard B, Friedenreich CM, Oppert JM, Clavel-Chapelon F. Effect of physical activity on women at increased risk of breast cancer: results from the E3N cohort study. Cancer Epidemiol Biomarkers Prev 2006;15:57–64. [DOI] [PubMed] [Google Scholar]
- Tehard B, van Liere MJ, Com Nougue C, Clavel-Chapelon F. Anthropometric measurements and body silhouette of women: validity and perception. J Am Diet Assoc 2002;102:1779–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripaldi R, Stuppia L, Alberti S. Human height genes and cancer. Biochim Biophys Acta 2013;1836:27–41. [DOI] [PubMed] [Google Scholar]
- Vitonis AF, Baer HJ, Hankinson SE, Laufer MR, Missmer SA. A prospective study of body size during childhood and early adulthood and the incidence of endometriosis. Hum Reprod 2010. a;25:1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitonis AF, Hankinson SE, Hornstein MD, Missmer SA. Adult physical activity and endometriosis risk. Epidemiology 2010. b;21:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle J, Carnell S, Haworth CM, Plomin R. Evidence for a strong genetic influence on childhood adiposity despite the force of the obesogenic environment. Am J Clin Nutr 2008;87:398–404. [DOI] [PubMed] [Google Scholar]
- Yi KW, Shin JH, Park MS, Kim T, Kim SH, Hur JY. Association of body mass index with severity of endometriosis in Korean women. Int J Gynaecol Obstet 2009;105:39–42. [DOI] [PubMed] [Google Scholar]