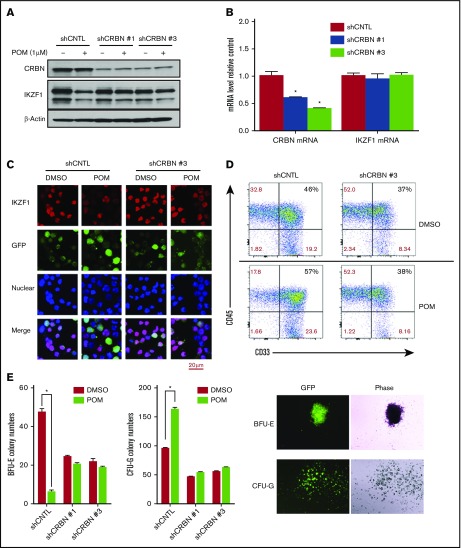
Figure 3.
CRBN mediates IMiD-induced IKZF1 degradation, resulting in a CD34+ cell lineage shift. CD34+ cells were transduced using a lentivirus with control shRNA (shCNTL), CRBN-shRNA#1 (shCRBN-1), or CRBN-shRNA#3 (shCRBN-3). The transfected cells were then sorted for GFP positivity 3 days after transduction. (A) GFP-sorted cells were treated with DMSO (0.01%) or POM (1 μM) for 6 hours. Cell lysates were analyzed by western blotting to compare the levels of CRBN and IKZF1. β-actin was used as a control. (B) The CRBN and IKZF1 mRNA levels in GFP-sorted CD34+ cells were analyzed by qRT-PCR. (C) shCNTL and shCRBN-3 CD34+ cells were treated with POM (1 μM) or DMSO (0.01%) for 6 hours. The treated cells were subsequently fixed with 4% formaldehyde and stained for IKZF1 (red color) or GFP (green color), using DAPI for nuclear counterstaining (blue color). The localization of IKZF1 was observed using a Leica microscope (200×). (D) To analyze myeloid differentiation, for GFP+, CD33+, and CD45+ were analyzed in shCNTL and shCRBN-3 CD34+ cells using flow cytometry. (E) Control (CNTL), shCRBN-1, and shCRBN-3 CD34+ cells were treated with DMSO (0.01%) or POM (1 μM) and used for the colony-formation assays to evaluate BFU-E and CFU-G colony formation. After 14 day, the colonies were counted using a Leica microscope (25×). *P ≤ .05.