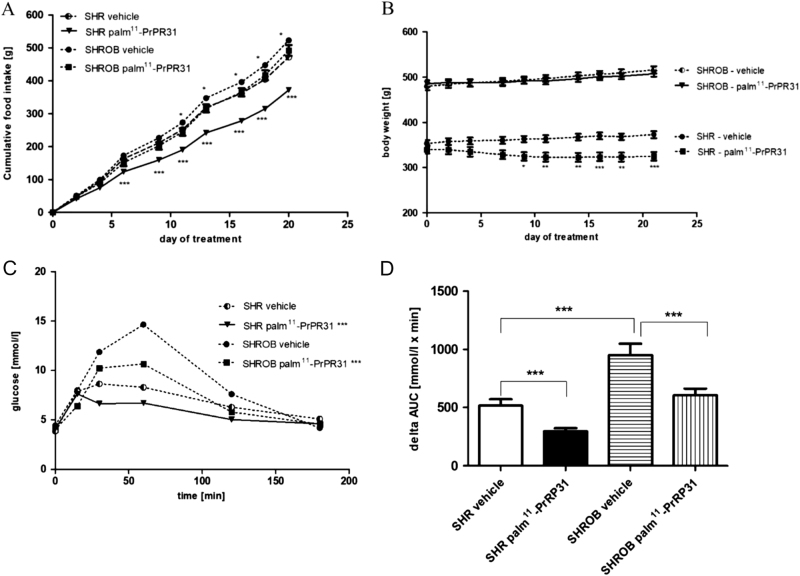
Fig. 2. Food intake, body weight and oral glucose tolerance test.
Chronic effect of palm11-PrRP31 on food intake (a), body weight (b), and oral glucose tolerance test (OGTT) response (c, d) in SHR and SHROB. palm11-PrPR31 was administered intraperitoneally at a dose of 5 mg/kg once a day for 21 days. Food intake and body weight were monitored every 2 days during drug application. OGTT was performed at the end of experiment; results are shown as glucose profile and delta AUC. Data are presented as means ± S.E.M. Statistical analysis was performed by repeated measures ANOVA with Bonferroni post hoc test, significance is *P < 0.05, **P < 0.01, ***P < 0.001 vs the vehicle-treated control group (n = 8)