Abstract
Background
Educational attainment (EA) is inversely associated with colorectal cancer risk. Colorectal cancer screening can save lives if precancerous polyps or early cancers are found and successfully treated. This study aims to estimate the potential productivity loss (PPL) and associated avoidable colorectal cancer–related deaths among screen-eligible adults residing in lower EA counties in the United States.
Methods
Mortality and population data were used to examine colorectal cancer deaths (2008–2012) among adults aged 50 to 74 years in lower EA counties, and to estimate the expected number of deaths using the mortality experience from high EA counties. Excess deaths (observed–expected) were used to estimate potential years life lost, and the human capital method was used to estimate PPL in 2012 U.S. dollars.
Results
County-level colorectal cancer death rates were inversely associated with county-level EA. Of the 100,857 colorectal cancer deaths in lower EA counties, we estimated that more than 21,000 (1 in 5) was potentially avoidable and resulted in nearly $2 billion annual productivity loss.
Conclusions
County-level EA disparities contribute to a large number of potentially avoidable colorectal cancer–related deaths. Increased prevention and improved screening potentially could decrease deaths and help reduce the associated economic burden in lower EA communities. Increased screening could further reduce deaths in all EA groups.
Impact
These results estimate the large economic impact of potentially avoidable colorectal cancer–related deaths in economically disadvantaged communities, as measured by lower EA.
Introduction
Colorectal cancer is a leading cause of suffering and cancer-related deaths in white, black, and Hispanic men and women in the United States (1). Each year, over 135,000 Americans are told they have colorectal cancer and more than 50,000 die from this disease. These numbers are expected to continue to increase as the population ages (2–3). Screening can reduce the number of incident cases and deaths by finding and removing preinvasive polyps, or by finding cancers at an early stage where treatment is more effective (4).
The risk of being diagnosed and dying from colorectal cancer increases in people aged 50 years and older (1). In 1995, the U.S. Preventive Services Task Force (USPSTF) recommended screening for colorectal cancer using FOBT or sigmoidoscopy among asymptomatic patients over the age of 50 years (5). These recommendations have evolved over time and have come to include recommendations for colonoscopy as well. In 2008, the USPSTF issued a revised recommendation that average risk adults 50 to 75 years of age be screened for colorectal cancer but concluded that evidence was insufficient to prioritize among screening tests (6). According to the Behavioral Risk Factor Surveillance System and National Health Interview Survey, the percentage of U.S. adults aged 50–75 years who self-reported receipt of recommended colorectal cancer screening tests has increased over the past decade in all racial/ethnic groups, and in all income and educational attainment groups (7–9). Screening rates were highest in women, whites, non-Hispanics, and those over the age of 65 years, and was positively associated with educational attainment and income.
Colorectal cancer incidence and death rates have been declining for the past several decades, likely the result of long-term declines in risk factors impacting incidence, with steeper declines since 2000, primarily attributed to screening (10). Declines in colorectal cancer incidence rates have not been equal in all racial, ethnic, and socioeconomic groups: among white Americans, incidence declined in both low and moderate poverty areas but not in high-poverty areas; among blacks, a modest decline was only seen in males living in low-poverty areas (11).
One measure of the economic burden of cancer is to estimate the potential years of life lost (PYLL), and the associated potential productivity loss (PPL) from these deaths. This study aims to estimate the economic burden resulting from these potentially avoidable deaths between 2008 and 2012 among 50–74 years old (USPSTF recommended age group) residents of lower educational attainment counties.
Materials and Methods
Mortality data were based on underlying cause of death information on death certificates filed in the 50 states and the District of Columbia (DC) and complied by the Centers for Disease Control and Prevention (CDC) National Vital Statistics System (12). For these analyses, we selected colorectal cancer deaths grouped by year of death (2008–2012). Population data were race-, ethnicity-, and sex-specific county population estimates from the 2010 U.S. Census and modified by the Surveillance, Epidemiology, and End Results (SEER) Program (13). Modifications incorporated bridged, single-race estimates that were derived from multiple-race categories and accounted for known issues in certain counties, including adjustment for populations displaced in Louisiana, Alabama, Mississippi, and Texas following hurricanes Katrina and Rita in 2005. Data from the U.S. American Community Survey files for 2008–2012 were used to derive measures of socioeconomic position (14). County-level educational attainment was based on the percentage of county residents, all races combined, aged ≥25 years, with at least a bachelor’s degree. We created equal population quintiles (Q1–5), with counties categorized by educational attainment as follows: Q1: ≥36.19%; Q2: 29.66–36.18%; Q3: 25.91–29.65; Q4: 18.88–25.90%; and Q5: <18.88%.
SEER*stat software (15) was used to calculate 5-year age-specific and age-standardized death rates per 100,000 population and rate ratios by sex and race/ethnicity [all races, non-Hispanic white (NHW), non-Hispanic black (NHB), and Hispanic]. Because of small numbers and lack of corresponding race-specific life tables, separate analyses were not performed for American Indian/Alaska Native or Asian or Pacific Islander, although these groups are included in the all races combined estimates. Analyses included data from all 50 states and the District of Columbia.
We described trends in age-standardized colorectal cancer death rate among people aged 50 and 74 years by county-level educational attainment quintile, sex, and grouped year of death. To estimate avoidable, excess deaths, we first estimated the number of expected colorectal cancer deaths between 2008 and 2012 by applying the 5-year age-specific death rate from counties with high educational attainment (Q1) to corresponding population estimates from counties with medium (Q2–3) and low (Q4–5) educational attainment. Excess deaths were calculated as observed minus expected deaths. Using the 2010 U.S. race/ethnicity- and sex-specific life tables (16), we used life expectancy to estimate PYLL which involved multiplying the number of excess deaths by the average remaining life expectancy in years among decedents. Because age at death was categorized, the midpoint of the 5-year age group was used as the descendants’ year of death. We then summed PYLL estimates to obtain overall PYLL estimates by race/ethnicity and age and sex.
We used the human capital approach to estimate lifetime mortality-related PPL (17). We estimated productivity loss by multiplying the number of excess deaths by the present value of (decedents’) future lifetime earnings. Estimated earnings including both market and household production, by 5-year age groups for men and women in the United States using data from the American Time Use Survey for year 2003. Hourly earnings and household production were used to derive estimates of first annual, then lifetime productivity. Estimates of earnings reflect factors such as life expectancy, the labor force participation rate, and the anticipated growth rate in productivity, and the imputed value of housekeeping services (e.g., cooking, cleaning, and child care). We applied these estimates based on a 3% discount rate (which was used to estimate the present value of future earnings estimates). We also conducted sensitivity analyses by applying estimates based on 0% and 5% discount rates. All estimated productivity was converted to 2012 U.S. dollars using the consumer price index (18).
Results
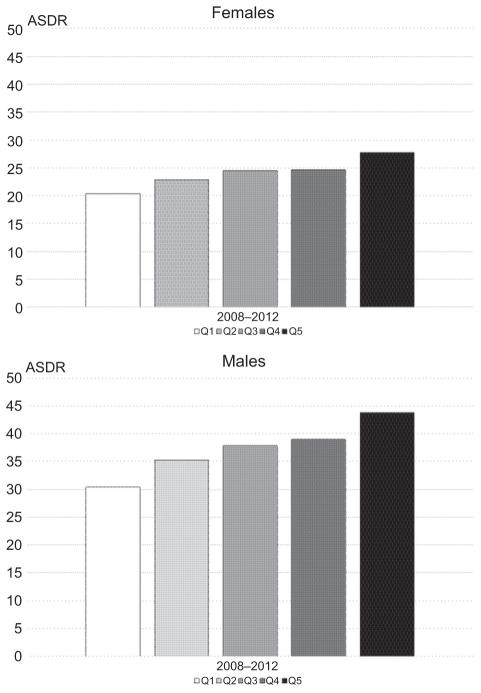
Between 2008 and 2012, colorectal cancer death rates among decedents aged 50–74 years were inversely associated with county-level education attainment in all major racial/ethnic groups (Fig. 1). Death rates were higher in men (37.5) compared with women (24.1) for all racial/ethnic groups; similar patterns were observed by race/ethnicity with the highest in NHB, followed by NHWs, and lowest in Hispanics (Table 1). Compared with decedents residing in high educational attainment (Q1) counties, rates were higher in medium (Q2–3) and highest in lower (Q4–5) educational attainment counties, in all racial/ethnic groups and both sexes, with the exception of Hispanic women where the colorectal cancer death rate in lower-educational attainment counties was not statistically significantly different from the colorectal cancer death in high educational attainment counties.
Figure 1.
Age-standardized colorectal cancer death rates (ASDR) among ages 50–74 years by county-level educational attainment* and sex, all races combined (2008–2012). NOTE: County-level educational attainment was categorized from high to low educational attainment according to the percentage of residents ages ≥25 years graduated college as follows: Q1, ≥36.19%; Q2, 29.66%–36.18%; Q3, 25.91%–29.65; Q4: 18.88%–25.90%; and Q5: <18.88%.
Table 1.
Age-standardized death rates per 100,000 and rate ratios (RR) by county-level educational attainment, sex, and race/ethnicity among colorectal cancer decedents ages 50–74 years (2008–2012)
| Educational attainmenta | All Races
|
NHW
|
NHB
|
Hispanic
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Deaths | Rate | Deaths | Rate | RR (95% CI) | Deaths | Rate | RR (95% CI) | Deaths | Rate | RR (95% CI) | |
| Males | |||||||||||
| High (Q1) | 10,451 | 30.4 | 7,635 | 29.2 | ref | 1,584 | 52.3 | ref | 567 | 25.2 | ref |
| Medium (Q2–3) | 25,652 | 36.5 | 17,375 | 34.3 | 1.18 (1.14–1.21) | 4,698 | 60.1 | 1.15 (1.08–1.22) | 2,473 | 32.4 | 1.29 (1.17–1.42) |
| Low (Q4–5) | 33,479 | 41.5 | 26,687 | 40.1 | 1.37 (1.34–1.41) | 4,254 | 63.2 | 1.21 (1.14–1.28) | 1,835 | 34.5 | 1.37 (1.24–1.51) |
| Total | 69,582 | 37.5 | 51,697 | 36.1 | 10,536 | 60.0 | 4,875 | 32.1 | |||
| Females | |||||||||||
| High (Q1) | 7,853 | 20.4 | 5,575 | 19.6 | ref | 1,298 | 33.1 | ref | 443 | 16.3 | ref |
| Med. (Q2–3) | 18,858 | 23.7 | 12,391 | 22.4 | 1.14 (1.10–1.18) | 3,891 | 38.2 | 1.16 (1.08–1.23) | 1,676 | 18.3 | 1.12 (1.01–1.25) |
| Low (Q4–5) | 22,868 | 26.2 | 18,084 | 25.6 | 1.30 (1.26–1.34) | 3,157 | 38.6 | 1.17 (1.09–1.25) | 1,028 | 17.4 | 1.07 (0.95–1.20) |
| Total | 49,579 | 24.1 | 36,050 | 23.3 | 8,346 | 37.4 | 3,147 | 17.7 | |||
County-level educational attainment was categorized from high to low educational attainment according to the percentage of residents ages ≥25 years graduated college as follows: Q1, ≥36.19%; Q2, 29.66%–36.18%; Q3, 25.91%–29.65%; Q4, 18.88%–25.90%; and Q5, <18.88% and grouped as medium educational attainment (Q2–3) and lower educational attainment (Q4–5).
The highest death rate was observed in NHB men (63.2) residing in counties with lower-educational attainment and lowest among Hispanic women (16.3) residing in counties with high educational attainment. Among NHB men, the rate in the high educational attainment counties (52.3) was greater than the rate in low educational attainment group in NHW men (40.1) and Hispanic men (34.5). Similar results were seen among women.
Table 2 shows age-standardized colorectal cancer death rates by race/ethnicity, sex; expected and excess colorectal cancer deaths and estimated PYLL among decedents aged 50 to 74 years who resided in counties with medium and lower-educational attainment, separately, and combined from 2008 through 2012. In total, 100,857 deaths (59,131 males and 41,726 females; Table 1) occurred in medium and low-educational attainment counties combined; 21,042 (13,336 males and 7,706 females) deaths were considered potentially avoidable. Proportionately more excess deaths occurred in men compared with women; and in counties with the low versus medium educational attainment, in all racial/ethnic groups with the exception of Hispanic women where women in medium educational attainment counties had more excess deaths than women in low-educational attainment counties.
Table 2.
Age-standardized death rates per 100,000, expected, excess deaths (%) in counties with medium- and low- educational attainment, among colorectal cancer decedents ages 50–74 years by sex, and race/ethnicity (2008 through 2012)
| Race/ethnicity | County-level educational attainment
|
|||||||
|---|---|---|---|---|---|---|---|---|
| High | Medium
|
Low
|
Middle and Low | |||||
| Rate | Rate | Exp. deaths | Excess deaths (%) | Rate | Exp. deaths | Excess deaths (%) | Excess deaths (%) | |
| Males | ||||||||
| All | 30.4 | 36.5 | 21,311 | 4,341 (16.9%) | 41.5 | 24,484 | 8,995 (26.9%) | 13,336 (22.6%) |
| NHW | 29.2 | 34.3 | 14,761 | 2,614 (15. 0%) | 40.1 | 19,403 | 7,284 (27.3%) | 9,898 (22.5%) |
| NHB | 52.3 | 60.1 | 4,080 | 618 (13.2%) | 63.2 | 3,505 | 749 (17.6%) | 1,367 (15.3%) |
| Hispanic | 25.2 | 32.4 | 1,921 | 552 (22.3%) | 34.5 | 1,331 | 504 (27.5%) | 1,056 (24.5%) |
| Females | ||||||||
| All | 20.4 | 23.7 | 16,207 | 2,651 (14.1%) | 26.2 | 17,813 | 5,055 (22.1%) | 7,706 (18.5%) |
| NHW | 19.6 | 22.4 | 10,842 | 1,549 (12.5%) | 25.6 | 13,890 | 4,194 (23.2%) | 5,743 (18.8%) |
| NHB | 33.1 | 38.2 | 3,392 | 496 (12.8%) | 38.6 | 2,696 | 461 (14.6%) | 960 (13.6%) |
| Hispanic | 16.3 | 18.3 | 1,487 | 189 (11.3%) | 17.4 | 959 | 69 (6.7%) | 258 (9.5%) |
Abbreviation: Exp., expected
Table 3 shows the PYLL was greater in males (276,169) than females (186,069) in each racial/ethnic group; and among residents of low-educational attainment counties compared with medium educational attainment counties with the exception of Hispanics and black women where PYLL were greater in the medium educational attainment counties.
Table 3.
PYLL and PPL in millions in counties with medium and low educational attainment among colorectal cancer decedents ages 50–74 years by sex and race/ethnicity (2008–2012)
| Race/ethnicity | County-level educational attainment
|
|||||
|---|---|---|---|---|---|---|
| Medium
|
Low
|
Medium and Low
|
||||
| PYLL | PPL 3% (5%, 0%) | PYLL | PPL 3% (5%, 0%) | PYLL | PPL 3% (5%, 0%) | |
| Males | ||||||
| All | 89,846 | $2.078 ($1.774–$2.757) | 186,323 | $4.315 ($3.683–$5.726) | 276,169 | $6.393 ($5.457–$8.483) |
| NHW | 53,236 | $1.194 ($1.021–$1.579) | 149,392 | $3.395 ($2.900–$4.498) | 202,628 | $4.590 ($3.921–$6.078) |
| NHB | 11,998 | $332 ($282–$444) | 14,801 | $419 ($356–$561) | 26,799 | $750 ($637–$1.005) |
| Hispanic | 12,764 | $297($252–$396) | 11,544 | $258 ($220–$343) | 24,308 | $555 ($473–$740) |
| Females | ||||||
| All | 64,482 | $1.230 ($1.024–$1.714) | 121,587 | $2.306 ($1.921–$3.212) | 186,069 | $3.536 ($2.946–$4.926) |
| NHW | 38,110 | $733 ($610–$1.023) | 100,853 | $1.914 ($1.594–$2.666) | 138,963 | $2.646 ($2.204–$3.689) |
| NHB | 10,703 | $209 ($174–$289) | 10,612 | $217 ($181–$302) | 21,314 | $426 ($355–$591) |
| Hispanic | 4,966 | $86 ($72–$118) | 1,995 | $37 ($31–$52) | 6,961 | $123 ($103–$170) |
NOTE: PPL expressed as millions.
Abbreviations: PPL, potential productivity loss; PYLL, potential years of life lost.
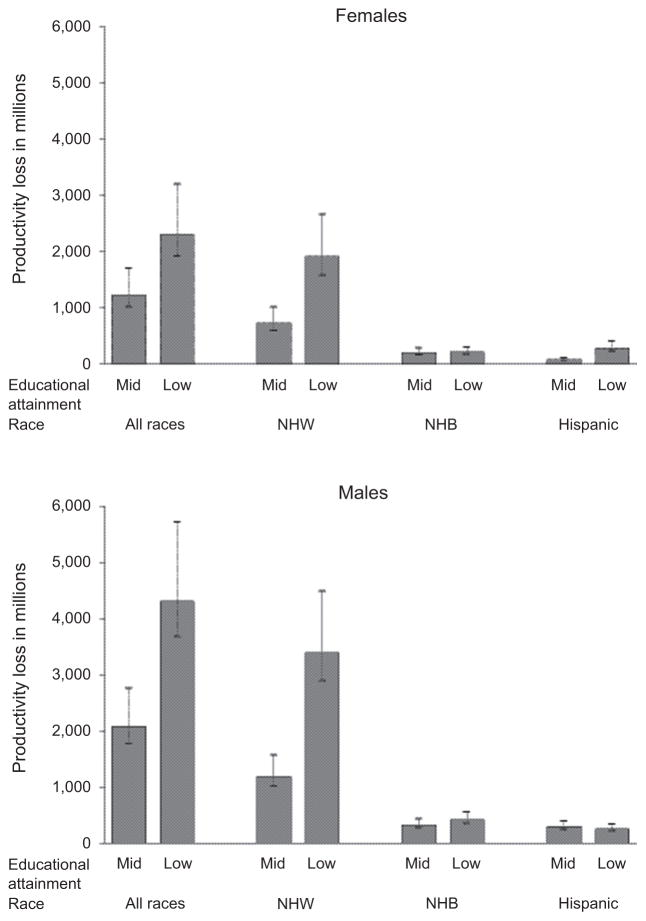
Table 3 and Fig. 2 show potential productivity loss (PPL) from potentially avoidable colorectal cancer deaths among men and women who resided in counties with medium and low-educational attainment between 2008 through 2012, separately and combined. Using a 0%, 3%, and 5% discount rate, combined PPLs were estimated to be $8,483 million, $6,393 million, and $5,457 million, respectively, in men; and $4,926 million, $3,536 million, and $2,946 million, respectively, in women. Estimates of PPL were higher in men compared with women; in NHWs compared with NHBs and lowest in Hispanics; and in residents of counties with low-educational attainment compared with medium educational attainment, with the exception of Hispanic men and women where the PPL was greater among decedents who resided in counties with medium educational attainment counties.
Figure 2.
Potential productivity loss (PPL) due to potentially avoidable excess colorectal cancer deaths among decedents ages 50–74 years in counties with medium-and low-educational attainment by sex and race/ethnicity (2008–2012). NOTE: County-level educational attainment was categorized from high to low educational attainment according to the percentage of residents ages ≥25 years graduated college as follows: Q1, ≥36.19%; Q2: 29.66%–36.18%; Q3, 25.91%–29.65; Q4, 18.88%–25.90%; and Q5, <18.88%.
Discussion
Colorectal cancer is a leading cause of premature death in the United States. Colorectal cancer death rates have decreased in men and women; however, as this study has shown, large socioeconomic disparities persist in all major racial and ethnic group, and represent a large number of potentially avoidable deaths. In this study, we estimated that of the 100,897 colorectal cancer deaths among decedents between 50 and 74 years of age that occurred in counties with medium and low-educational attainment, more than 21,000 deaths (just over 1 in 5), may have been related to factors associated with lower educational attainment. These deaths resulted in approximately $9.9 billion dollars in lost productivity over a 5-year period, or a nearly a 2 billion dollar annually loss. Productivity losses due to cancer mortality are large and growing (19–20) and, as this study has shown, disproportionately impact counties that are already economically disadvantaged, as these decedents are no longer contributing to the economic wellbeing of their families or communities.
In the early 1980s, colorectal cancer death rates were more common among the affluent but toward the end of the last century, death rates decreased in more affluent groups (21, 22). As a result, colorectal cancer death rates are now more common in the less affluent groups. A number of health behaviors such as consumption of red meat, alcohol, smoking and lack of physical activity, and chronic conditions such as obesity and diabetes have been shown to raise the risk of developing colorectal cancer (23, 24) and been shown to be associated with educational attainment and income (25). In the most recent time period (2008–2012), the lower colorectal cancer death rates among higher socioeconomic groups may reflect the impact of colorectal cancer screening. While screening rates have increased in all socioeconomic groups, rates actually increased the most in higher socioeconomic group (5, 8–9, 26). Consistent with this observation, there was a decline in late-stage colorectal cancer incident cases in white men and women residing in low and moderate poverty areas between 1992 and 2004 but no decline among white men and women residing in high-poverty areas (11).
Black men and women have higher rates of colorectal cancer incidence and deaths than whites (1). In our study, the racial disparities were stark: black men residing in low socioeconomic counties had a colorectal cancer death rate that was nearly four times higher than the rate observed among Hispanic women residing in high socioeconomic counties; and black men and women residing in high educational attainment counties had higher colorectal cancer death rates than white men and women residing in low education attainment counties. Microsimulations of mortality data have been used to explore the racial disparities in colorectal cancer death rates and found that approximately half of the differences could be explained by lower screening rates and lower stage-specific survival (27). Screening rates between white and blacks have narrowed in recent years (7–9). However, it may take years before the gap in mortality rates begins to narrow, if at all. Disparities may remain because of differences in stage-specific survival which appear to be the result of differences in access to high quality health care (28). Therefore, eliminating potentially avoidable premature deaths will depend on additional factors including, but not limited to, timely follow-up of patient with positive tests, and initiation and completion of evidence-based treatment.
These socioeconomic and racial disparities represent a large number of potentially avoidable premature deaths. Our methods for estimating potentially avoidable deaths were straightforward. Over the 5-year period, we estimated that of all colorectal cancer deaths, approximately 27% (8,995) in males and 22% (5,055) in females residing in low educational attainment counties and approximately 17% (4,341) in males and 14% (2,651) females residing in medium educational attainment counties were potentially avoidable. Higher percentages of excess deaths were estimated in low educational attainment counties among all major racial and ethnic groups with the exception of Hispanic women where a higher percentage of excess deaths was estimated among residents of medium educational attainment counties. These findings are based on recent U.S. vital statistics data which are considered reliable for reporting Hispanic origin (29).
The distribution of colorectal cancer deaths in the population is determined by the underlying risk of dying within the population and the size and age structure of the population. Geospatial analyses has been used to identify areas of the United States with usually high age-adjusted rates (risk) of colorectal cancer deaths (30). Combining risk of death with estimates of excess deaths might help target screening and treatment interventions so as to achieve maximum impact.
An even larger number of colorectal cancer deaths could likely be avoided if screening rates were increased to meet the Healthy People 2020 target for colorectal cancer screening (70.5% of adults aged 50 to 75 years received a colorectal cancer screening; ref. 31). The National Colorectal Cancer Roundtable initiated a more ambitious goal to increase colorectal cancer screening rates to 80% by 2018 (32). According to their estimates, achieving this goal could potentially avert approximately 203,000 premature deaths between 2013 and 2030 (33) or approximately 11,000 colorectal cancer deaths per year. And further benefit might follow from re-evaluating age-based screening guidelines and improving screening completion among older adults (34). The USPSTF has just recently updated their guidance regarding colorectal cancer screening in adults aged 76 to 85 years, noting that screening would be most appropriate among older adults who are healthy enough to undergo treatment if colorectal cancer is detected and do not have comorbid conditions that would substantially limit their life expectancy (35).
Long-term, increased efforts aimed at the primary prevention of colorectal cancer, such as reduction of exposure to known colorectal cancer risk factors (23–24) and health promotion among colorectal cancer survivors (36), would further reduce colorectal cancer death.
Strengths and Limitation
Our estimates of potentially avoidable deaths are conservative compared to the study by Jemal and colleagues (35) in which it was estimated that approximately half of colorectal cancer deaths among those between the ages of 24 and 64 years could have been prevented if the death rate in each racial and ethnic group was as low (i.e., favorable) as the rate observed in the most educated non-Hispanic white group in the five states with the lowest colorectal cancer death rates. Such an estimate presents a best case scenario.
Our study employed an ecologic design and used county-level educational attainment to estimate excess colorectal cancer deaths in lower educational-attainment counties. We chose to use educational-attainment because of its positive association with screening, which has been shown to reduce both colorectal cancer incidence and death rates. County was the smallest area-based measure available in the National Vital Statistics System. County measures are a crude proxy for an individual’s educational attainment because there may be a lack of uniformity among residents living in the same county.
Estimates of the value of lost productivity that resulted from potentially avoidable colorectal cancer deaths includes the value of future lost salaries and wages, and the value of household activities such as cooking, cleaning, and child care. As such, these estimates reflect the additional economic burden born by counties that are already economically disadvantaged. Our estimates are based on deaths and do not include other indirect costs or monetary losses associated with time spent receiving medical care, time lost from work, that is, lost productivity associated with morbidity. These costs are incurred by informal caregivers and families as well as patients. Examining the costs associated with diagnosing and treating these cancers would contribute to a more complete picture of the overall economic benefit if routine screening, diagnosis, and follow-up care were available to all screen-eligible men and women residing in lower socioeconomic communities.
Acknowledgments
Grant Support
This work was supported by the Division of Cancer Prevention and Control at the National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Disc laimers
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Authors’ Contributions
Conception and design: H.K. Weir, C. Li
Development of methodology: H.K. Weir, C. Li
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): H.K. Weir, C. Li, S.J. Henley, D. Joseph
Writing, review, and/or revision of the manuscript: H.K. Weir, C. Li, S.J. Henley, D. Joseph
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): C. Li
Study supervision: H.K. Weir
References
- 1.U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2013 Incidence and Mortality Web-based Report. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2016. Available from: www.cdc.gov/uscs. [Google Scholar]
- 2.Weir HK, Thompson TD, Soman A, Moller B, Leadbetter S. The past, present, and future of cancer incidence in the United States: 1975 through 2020. Cancer. 2015;121:1827–37. doi: 10.1002/cncr.29258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weir HK, Thompson TD, Soman A, Moller B, Leadbetter S, White MC. Meeting the healthy people 2020 objectives to reduce cancer mortality. Prev Chronic Dis. 2015;12:E104. doi: 10.5888/pcd12.140482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Rockville, MD: U.S. Preventive Services Task Force; 2008. Available from: http://www.uspreventiveservicestaskforce.org/uspstf08/colocancer/colors.htm. [Google Scholar]
- 5.Levin B, Bond JH. Colorectal cancer screening: recommendations of the U.S. Preventive Services Task Force. American Gastroenterological Association. Gastroenterology. 1996;111:1381–84. doi: 10.1053/gast.1996.1111381. [DOI] [PubMed] [Google Scholar]
- 6.Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:638–58. doi: 10.7326/0003-4819-149-9-200811040-00245. [DOI] [PubMed] [Google Scholar]
- 7.Steele CB, Rim SH, Joseph DA, King JB, Seff LC. Colorectal cancer incidence and screening - United States, 2008 and 2010. MMWR Suppl. 2013;62:53–60. [PubMed] [Google Scholar]
- 8.Rim SH, Joseph DA, Steele CB, Thompson TD, Seff LC. Colorectal cancer screening - United States, 2002, 2004, 2006, and 2008. MMWR Suppl. 2011;60:42–6. [PubMed] [Google Scholar]
- 9.Sabatino SA, White MC, Thompson TD, Klabunde CN Centers for disease C, prevention. Cancer screening test use - United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64:464–68. [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–73. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegel RL, Jemal A, Thun MJ, Hao Y, Ward EM. Trends in the incidence of colorectal cancer in relation to county-level poverty among blacks and whites. J Natl Med Assoc. 2008;100:1441–44. doi: 10.1016/s0027-9684(15)31544-3. [DOI] [PubMed] [Google Scholar]
- 12.National Vital Statistics System (NVSS) Centers for Disease Control and Prevention; 2014. Available from: http://www.cdc.gov/nchs/nvss.htm. [Google Scholar]
- 13.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, et al., editors. SEER Cancer Statistics Review, 1975–2013. National Cancer Institute; Bethesda, MD: Apr, 2016. Available from: http://seer.cancer.gov/csr/1975_2013/ based on November 2015 SEER data submission, posted to the SEER web site. [Google Scholar]
- 14.National Cancer Institute. Surveillance Epidemiology and end results program. County attributes. 2015 Available from: http://seer.cancer.gov/seerstat/variables/countyattribs/#08-12.
- 15.Surveillance Research Program. SEER*Stat software (version 482.0.1) Available from: http://seer.cancer.gov/seerstat/
- 16.United States Life Tables. 2010 Available from: http://www.cdc.gov/nchs/data/nvsr/nvsr63/nvsr63_07.pdf. [PubMed]
- 17.Grosse SD, Krueger KV, Mvundura M. Economic productivity by age and sex: 2007 estimates for the United States. Med Care. 2009;47:S94–103. doi: 10.1097/MLR.0b013e31819c9571. [DOI] [PubMed] [Google Scholar]
- 18.Consumer Price Index. Bureau of Labor Statistics; Available from: http://www.bls.gov/cpi/ [Google Scholar]
- 19.Bradley CJ, Yabroff KR, Dahman B, Feuer EJ, Mariotto A, Brown ML. Productivity costs of cancer mortality in the United States: 2000–2020. J Natl Cancer Inst. 2008;100:1763–70. doi: 10.1093/jnci/djn384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yabroff KR, Mariotto AB, Feuer E, Brown ML. Projections of the costs associated with colorectal cancer care in the United States, 2000–2020. Health Econ. 2008;17:947–59. doi: 10.1002/hec.1307. [DOI] [PubMed] [Google Scholar]
- 21.Singh GK, Miller BA, Hankey BF, Feuer EJ, Pickle LW. Changing area socioeconomic patterns in U.S. cancer mortality, 1950–1998: Part I–All cancers among men. J Natl Cancer Inst. 2002;94:904–15. doi: 10.1093/jnci/94.12.904. [DOI] [PubMed] [Google Scholar]
- 22.Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural-urban, and racial inequalities in US cancer mortality: part I-all cancers and lung cancer and part II-colorectal, prostate, breast, and cervical cancers. J Cancer Epidemiol. 2011;2011:107497. doi: 10.1155/2011/107497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouvard V, Loomis D, Guyton KZ, Grosse Y, Ghissassi FE, Benbrahim-Tallaa L, et al. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015;16:1599–1600. doi: 10.1016/S1470-2045(15)00444-1. [DOI] [PubMed] [Google Scholar]
- 24.Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology. 2010;138:2029–43. doi: 10.1053/j.gastro.2010.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doubeni CA, Laiyemo AO, Major JM, Schootman M, Lian M, Park Y, et al. Socioeconomic status and the risk of colorectal cancer: an analysis of more than a half million adults in the national institutes of health-AARP diet and health study. Cancer. 2012;118:3636–44. doi: 10.1002/cncr.26677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klabunde CN, Cronin KA, Breen N, Waldron WR, Ambs AH, Nadel MR. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20:1611–21. doi: 10.1158/1055-9965.EPI-11-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lansdorp-Vogelaar I, Kuntz KM, Knudsen AB, van Ballegooijen M, Zauber AG, Jemal A. Contribution of screening and survival differences to racial disparities in colorectal cancer rates. Cancer Epidemiol Biomarkers Prev. 2012;21:728–36. doi: 10.1158/1055-9965.EPI-12-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soneji S, Iyer SS, Armstrong K, Asch DA. Racial disparities in stage-specific colorectal cancer mortality: 1960–2005. Am J Public Health. 2010;100:1912–16. doi: 10.2105/AJPH.2009.184192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arias E. United States life tables by Hispanic origin. Vital Health Stat 2. 2010;152:1–33. [PubMed] [Google Scholar]
- 30.Siegel RL, Sahar L, Robbins A, Jemal A. Where can colorectal cancer screening interventions have the most impact? Cancer Epidemiol Biomarkers Prev. 2015;24:1151–56. doi: 10.1158/1055-9965.EPI-15-0082. [DOI] [PubMed] [Google Scholar]
- 31.About Healthy People. Washington (DC): US Department of Health and Human Services; 2012. [Accessed October 22, 2014]. Available from: http://www.healthypeople.gov/2020/About-Healthy-People. [Google Scholar]
- 32.National Colorectal Cancer Roundtable 80% by 2018. Available from: http://nccrt.org/tools/80-percent-by-2018/
- 33.Meester RG, Doubeni CA, Zauber AG, Goede SL, Levin TR, Corley DA, et al. Public health impact of achieving 80% colorectal cancer screening rates in the United States by 2018. Cancer. 2015;121:2281–85. doi: 10.1002/cncr.29336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klabunde CN, Zheng Y, Quinn VP, Beaber EF, Rutter CM, Halm EA, et al. Influence of age and comorbidity on colorectal cancer screening in the elderly. Am J Prev Med. 2016;51:e67–75. doi: 10.1016/j.amepre.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.U.S. Preventive Services Task Force. Colorectal Cancer: Screening Release Date. 2016 Available from: http://www.uspreventiveservices-taskforce.org/Page/Document/UpdateSummaryFinal/colorectal-cancer-screening2.
- 36.El-Shami K, Oeffinger KC, Erb NL, Willis A, Bretsch JK, Pratt-Chapman ML, et al. American cancer society colorectal cancer survivorship care guidelines. CA Cancer J Clin. 2015;65:428–55. doi: 10.3322/caac.21286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jemal A, Siegel RL, Ma J, Islami F, DeSantis C, Goding Sauer A, et al. Inequalities in premature death from colorectal cancer by state. J Clin Oncol. 2015;33:829–35. doi: 10.1200/JCO.2014.58.7519. [DOI] [PubMed] [Google Scholar]