Abstract
Background
Individuals with a history of maltreatment show altered amygdala reactivity to emotional stimuli, atypical frontal regulatory control, and differences in frontolimbic connectivity compared to non-maltreated controls. However, despite early trauma, many individuals who experience maltreatment show resilience, or adaptive functioning in adulthood including positive social, educational, and occupational outcomes.
Methods
The present study employed a psychophysiological interaction (PPI) model to examine the effect of adult adaptive functioning on group differences between maltreated and non-maltreated adults in task-based amygdala functional connectivity. The task employed was a facial emotion-matching paradigm. fMRI scans were collected from 41 adults with a history of substantiated childhood maltreatment and 39 non-maltreated adults who were well matched on demographic variables, all of whom had been studied since childhood. Adaptive functioning was measured with a composite score of success on stage-salient developmental tasks.
Results
Consistent with prior research, we found differences in task-related amygdala functional connectivity between the maltreated and non-maltreated groups. Effects were seen in the left hippocampus, right dorsolateral prefrontal cortex, dorsomedial prefrontal cortex, and right thalamus. However, when adult functioning was included in the model, maltreatment-related differences in amygdala connectivity were observed only in the hippocampus. Adult adaptive functioning independently predicted task-related amygdala connectivity in frontal and parietal regions across the entire sample.
Conclusions
These results suggest that frontolimbic functional connectivity is predicted by positive developmental adaptation in this high-risk population, regardless of maltreatment history, whereas intra-limbic connectivity (amygdala and hippocampus) is more specifically associated with maltreatment history.
Keywords: Childhood adversity, Maltreatment, Adaptive functioning, Neuroimaging, Frontolimbic connectivity, Amygdala
Childhood maltreatment (CM) poses significant risks of biological and psychological maladaptation, and increases the likelihood of psychopathology across the lifespan (1). Emotional reactivity and emotion processing are often affected in individuals with a history of CM (2). For instance, deficits in emotion recognition, response biases to angry facial expressions, and delayed disengagement from such stimuli have been noted in this population during childhood and adulthood (4–10). This persistent vigilance for negative facial expressions may help maltreated individuals detect and thereby avoid angry confrontations with their abusive caregivers; however, such hypersensitivity has been linked to maladaptive behavior outside of the context of CM, when there is no longer an imminent threat of abuse (11).
Extensive evidence has established a heightened response of the amygdala to threat-related cues in individuals with posttraumatic stress disorder (PTSD) as well as in adults with histories of childhood emotional abuse or neglect, independent of psychiatric status (12, 13). The prefrontal cortex (PFC), a region known to be involved in cognitive and behavioral control processes, has also been identified as functionally aberrant following early adversity and CM (14, 15). Of course, these regions (amygdala and PFC) are known to be structurally and functionally connected (16). Connectivity within frontolimbic circuits has been related to efficient emotion regulation, fear conditioning and fear extinction (17, 18). The strength of functional connectivity (FC), or the degree to which regions within the emotion processing network co-activate, has recently been shown to be altered in individuals who experienced CM, both when measured at rest (19) and also when performing face emotion-processing tasks (20, 21). Such work, including our own (21), has shown that adults with a history of CM show greater emotion-related frontolimbic connectivity in comparison to those without CM, which may indicate an inefficient regulatory system.
However, studies of maltreated samples generally do not consider individual differences that may affect long-term outcomes following early adversity. Furthermore, group comparisons can mask individual differences in the capacity for resilience, or dynamic and positive adaptation in the face of significant adversity (22). Early work has shown that even in the face of chronic and severe adversity, most children have the capacity for resilient outcomes in one or more domains (23, 24). However, there have been few studies on the neurobiological correlates of resilience. One study assessed effortful modification of emotional responses to negative pictures, and another measured resting-state functional connectivity. These two small studies have found that, compared to both vulnerable trauma-exposed and non-trauma-exposed individuals, resilient trauma-exposed individuals show differential frontal brain activity (25, 26). Yet, it remains unknown whether or not resilience, the process of, capacity for, or outcomes of adaptive functioning despite threat, may moderate differences in task-related frontolimbic FC that have been attributed to CM (27).
To fill this gap in the literature and build upon our previous work on frontolimbic connectivity following CM (21), we evaluated the effects of CM history and adult adaptive functioning on amygdala connectivity in adults performing a face emotion-processing task. Adults with a verified CM history and a matched comparison group were rated on an adaptive functioning scale to approximate resilient processes and completed an emotional faces task known to elicit limbic activation during functional magnetic resonance imaging (fMRI) (28). Psychophysiological interaction (PPI) connectivity analyses were performed to investigate differential amygdala connectivity to emotional and neutral stimuli in those with and without a history of CM. In light of the aforementioned findings of 1) greater frontolimbic FC in CM adults (20, 21), and 2) differences in neural processing between resilient trauma-exposed individuals and patients with trauma-related psychopathology (25, 26, 29), we predicted that both maltreatment status and adult adaptive functioning would correlate with emotion-related amygdala connectivity. Specifically, we expected that individuals at highest risk (prior CM and low adaptive functioning scores) would show increased emotion-related frontolimbic connectivity compared to lower risk individuals (SES-matched non-maltreated or CM with high adaptive functioning scores).
Methods and Materials
Participants
Participants included 80 adults who were part of a longitudinal sample, first recruited through a research summer camp for low-income, high-risk children when they were 6 –12 years of age, and subsequently assessed once or twice during adolescence prior to the current adult assessment (30). All children were from low-income families, with 93% reporting a history of receiving public assistance. Forty-one participants had a history of CM as documented by Department of Human Services (DHS) records, and 39 other participants were classified as non-CM via lack of DHS records through age 17 years. The Maternal Maltreatment Classification Interview was used to further verify CM history, or the lack thereof. Comprehensive DHS records were coded using the Maltreatment Classification system to classify the type and developmental timing of each report of substantiated maltreatment (31). The majority (70%) the CM group experienced more than one type of maltreatment. Depressive or general internalizing symptoms (as measured by the Adult Self Report measure and BDI-II; 32, 33) did not differ by group. Demographic information for the final sample is provided in Table 1. All participants provided informed consent in compliance with the University of Rochester’s Institutional Review Board and were compensated for their time. A subset of these data was reported previously by our group (21).
Table 1.
Demographics and sample characteristics for the maltreated and comparison groups
| Sample Characteristics | Maltreated Group (N = 41) | Comparison Group (N = 39) | p-value (2-tailed) |
|---|---|---|---|
| Age (years), M (SD) | 30.85 (3.28) | 29.33 (3.53) | .049 |
| Male, n (%) | 18 (43.9) | 23 (59.0) | .182 |
| Race, n (%) | .257 | ||
| Black | 24 (58.5%) | 29 (74.4%) | |
| White | 12 (29.3%) | 5 (12.8%) | |
| Other/multiracial | 5 (12.2%) | 5 (12.8%) | |
| Total annual family income, | $30.60k ($22.31k) | $32.56k ($25.22k) | .713 |
| M (SD) | |||
| Range | $2.3k – $103.0k | $5.2k – $120.0k | |
| Marital status, n (%) | .688 | ||
| Not married | 35 (85.4) | 32 (82.1) | |
| Married | 6 (14.6) | 7 (17.9) | |
| Current work status, n (%) | |||
| Working full time | 19 (46.3) | 20 (51.3) | .845 |
| Working part time | 8 (19.5) | 8 (20.5) | |
| Not working | 14 (34.1) | 11 (28.2) | |
| Education, n (%) | .203 | ||
| Some high school | 8 (19.5) | 4 (10.3) | |
| High school diploma or GED | 15 (36.6) | 17 (43.6) | |
| Tech degree, associates degree, or some college | 13 (31.7) | 17 (43.6) | |
| Bachelor’s or master’s degree | 5 (12.2) | 1 (2.6) | |
| Adult adaptive functioning, M (SD) | 6.61 (3.06) | 7.38 (2.75) | .240 |
| Beck Depression Inventory- II total score, M (SD) | 10.15 (9.56) | 10.13 (8.16) | .993 |
| Adult Self Report | 50.83 (10.77) | 51.67 (12.32) | .747 |
| Internalizing t-score, M (SD) | |||
| Maltreatment type, n (%) | |||
| Emotional maltreatment | 23 (59.0) | - | |
| Physical neglect | 31 (79.5) | - | |
| Physical abuse | 21 (53.8) | - | |
| Sexual abuse | 7 (17.9) | - | |
| Maltreatment onset, n (%) | |||
| Infancy | 19 (48.7) | - | |
| Toddlerhood | 7 (17.9) | - | |
| Preschool | 6 (15.4) | - | |
| Early school | 1 (2.6) | - | |
| Later school | 4 (10.3) | - |
Note: Statistical effects were tested by ANOVA or t-test as appropriate for the number of levels. For two participants with a history of child maltreatment, there was insufficiently detailed data to classify subtype and onset.
Data collected from an additional 23 individuals were excluded from the final sample due to: task accuracy more than 2 SD below the overall mean on control trials (<75% correct; 5 CM, 1 comparison); serious mental illness identified by history of hospitalization (2 CM: 1 schizophrenia, 1 bipolar disorder); structural brain anomalies (2 CM, 3 comparison); or excessive head motion (6 CM, 4 comparison). An additional 53 longitudinal participants were contacted and screened, but were unable to participate due to incarceration, death, scheduling conflicts, MRI contraindications, or decline to participate (see Supplement 1 for details).
Measure of Adult Adaptive Functioning
Contemporary conceptions of resilience align with a developmental psychopathology perspective that focuses on both negative and positive adaptation in response to stress. These adaptations can be measured along dimensions within various domains and at multiple levels of analysis (34, 35). Therefore, we measured success on stage-salient developmental tasks to approximate resilient outcomes. A developmental task is a task typical to a certain period of life for which successful achievement leads to competence and later successes, while failure leads to incompetence in the individual, disapproval by society, and difficulty with later tasks (36). Developmental tasks in early adulthood have been shown to predict competence in adults over a 10-year period (36, 37).
We used a composite (range 0 to 14) of rank scores based on participants’ progress in seven domains of development: education, work, financial autonomy, romantic involvement, peer involvement, family involvement, and substance abuse. This approach was based on work by Schulenberg and colleagues (37). Information from each domain was drawn from the Adult Self Report measure (32) and a demographics questionnaire. Participants were ranked in one of three categories for each domain based on their success on the developmental task relative to other participants in the study. Therefore, successful development was defined in relation to others from similar economic and social backgrounds. For each domain, rankings were based on cut-offs that approximately divided the participants into thirds (lowest, middle, and highest third). Details can found in Supplement 1.
Behavioral fMRI Paradigm
In the scanner, participants were asked to perform a matching task of trials containing either emotionally expressive faces or simple geometric shapes. On each trial, they were required to indicate which of two stimuli (faces or shapes) presented at the bottom of the screen matched the stimulus at the top of the screen. The face stimuli were black-and-white photographs of angry and fearful expressions selected from a standardized set of emotional faces (38). For face trials, participants matched based on emotional expression, as each face trial included expressions posed by three different actors of the same sex. Shape stimuli were solid black shapes (circles, or horizontal or vertical ellipses) presented on a white background. The task consisted of a total of nine blocks, each containing six trials, with alternating blocks of shape and emotion matching (five and four blocks, respectively). Stimuli were presented for 4500 ms, with a 500 ms interstimulus interval.
MRI Acquisition
Details concerning data acquisition are presented in Supplement 1.
fMRI Data Analysis
Preprocessing
MRI data were analyzed using FSL (FMRIB Software Library, v. 5.0.8; 39). Preprocessing involved motion correction with MCFLIRT (Motion Correction FMRIB’s Linear Image Registration Tool), skull stripping using the Brain Extraction Tool, slice timing correction, geometric unwarping based on a fieldmap volume, spatial smoothing using a 6-mm FWHM Gaussian kernel, and high-pass temporal filtering with a filter cutoff of 60 s based on task design. Motion displacement was quantified using the root mean square across the six motion correction parameters. Volumes were assessed for censoring based on the following parameters: (a) absolute motion exceeding one voxel of overall displacement from the first volume in the series and (b) relative motion exceeding one-half voxel from one volume to the next. Volumes immediately preceding and following those that met the relative criterion were also excluded. Participants were excluded if the number of TRs censored exceeded 25% of the scan, meaning that all participants had at least 107 TRs, or 3 min 35 sec of usable data. Finally, each participant’s functional images were registered to the corresponding high-resolution anatomical image (using 6 df), which was in turn ryegistered to MNI standard space (Montreal Neurological Institute’s MNI 152 T1 2-mm template; using 12 df).
Task Analysis
Individual data were entered into a general linear model (GLM) using a gamma-convolved predictor for emotion-matching blocks, with shape matching as the unmarked baseline. Additional predictors of non-interest included 1 predictor for buffer trials (short fixation periods at the beginning and end of the task), 6 predictors for motion (3 rotation and 3 linear translation), and motion displacement (censoring) predictors for each affected TR.
Group Analyses
A whole-brain regression analysis was run to find group differences between the maltreated and comparison group, with age, sex, and accuracy on emotion-matching trials as nuisance variables.
Psychophysiological Interaction (PPI) Analysis
PPI analyses were conducted to examine effects of CM and adaptive functioning on task-based amygdala FC during emotion processing (40, 41). Whole-brain analyses were run using the bilateral amygdala as the seed region. This analysis identified regions where activation was temporally correlated with activation in the bilateral amygdala during the emotion-matching component of the task relative to the shape-matching baseline. We created our bilateral amygdala mask based on the Harvard–Oxford subcortical anatomical atlas included with FSL (HarvardOxford-sub-maxprob-thr0–2mm.nii.gz), and dilated the mask by 3 mm to ensure adequate coverage across individual variations in anatomy. We obtained the bilateral amygdala signal for each participant by back-projecting this mask into each participant’s original functional data space and extracting the mean physiological time series from within the mask. An interaction predictor was created to identify regions that covaried in a task-dependent manner with amygdala activity. Specifically, this PPI regressor was obtained by multiplying the gamma-convolved emotion predictor (zero-centered) by the demeaned time-series of the bilateral amygdala ROI. Single subject GLM models included: the physiological time series from the bilateral amygdala, the gamma-convolved emotion and buffer predictors, and the PPI predictor (amygdala signal x emotion predictor), as well as six head motion predictors (3 rotation and 3 linear translation), and predictors for each censored time point.
Group Analyses
Three higher level (group) analyses were conducted to evaluate whether amygdala networks were modulated by CM and adaptive functioning (mean centered). First, a whole-brain regression analysis was run to find group differences between the maltreated and comparison group without controlling for the influence of adaptive functioning. Next, a whole-brain regression analysis was run with group and adaptive functioning score as predictors. This allowed us to examine group differences while controlling for the effect of adaptive functioning, and vice versa. Finally, a third whole-brain regression analysis was run that mimicked the second, but with the addition of the interaction term. All analyses included age, sex, and accuracy for the emotion-matching condition (all mean-centered) as nuisance variables. In all fMRI analyses, significance was assessed using FSL’s cluster correction procedure with a voxelwise threshold of p <.005 and a cluster threshold of p < .05.
Results
Behavioral Results
As expected, performance was poorer (lower accuracy and longer response time) for the emotion-matching compared to the shape-matching condition in the whole sample. Between groups, accuracy was lower for the emotion-matching condition in the CM group relative to the non-CM group. The two groups did not differ in performance on the shape trials, nor did they differ in response time for emotion-matching or shape-matching conditions.
Adult adaptive functioning, as measured by developmental task scores, did not differ between groups (CM and non-CM). It also did not relate to the number of developmental periods maltreatment was sustained. Adaptive functioning also did not relate to accuracy or response time on either the shape-matching or emotion-matching trials. Adaptive functioning was positively associated with age, and differed by sex, with females showing higher developmental task scores than males. Accordingly, all PPI analyses included age, sex, and accuracy for the emotion-matching condition as demeaned covariates. Detailed behavioral results can be found in Behavioral Results of Supplement 1.
Task Reactivity
An analysis of reactivity to the task in the whole sample revealed significant activation (emotion matching > shape matching) in multiple brain regions, including bilateral amygdala, medial frontal gyrus, middle frontal gyrus, thalamus, basal ganglia, and occipital lobe. These regions are consistent with those reported in previous studies using this task (28, 42). There were no group differences in reactivity to the task effect after cluster correction. Effects of adaptive functioning are noted in Supplement 1.
PPI Effects: Task-based Amygdala Connectivity
As a first step in PPI analyses, we examined general patterns of emotion-related amygdala connectivity across all subjects. Significant emotion-related FC with the amygdala was observed in the medial prefrontal gyrus, anterior cingulate cortex, middle frontal gyrus, caudate, insula, posterior cingulate, thalamus, hippocampus and parahippocampal gyrus.
PPI Effects: Group Differences (CM vs. non-CM)
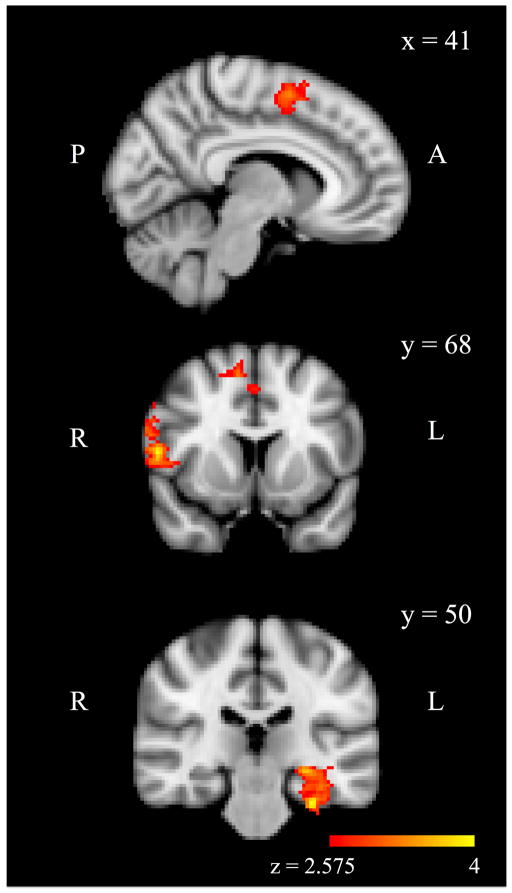
Next, to parallel prior studies of maltreatment including our own with a subset of this sample (21), we evaluated patterns of amygdala connectivity that differed by group. Three regions showed a main effect of group with greater PPI values for the CM group than the non-CM group (Figure 1). These regions included the left hippocampus and parahippocampal gyrus, right dorsolateral prefrontal cortex, and dorsomedial prefrontal cortex. There were no regions that showed lower PPI values in the CM group than in the non-CM group.
Figure 1.
Regions in which bilateral amygdala-based psychophysiological interaction (PPI) (emotion-matching > shape-matching) was greater for the childhood maltreatment group than the comparison group, p < .05 cluster corrected.
PPI Effects: Group Differences (CM vs. non-CM, Controlling for Adaptive Functioning)
We followed the initial group analysis with a parallel analysis including adaptive functioning in the model. Analysis of amygdala-based emotion-related PPI connectivity revealed that the left hippocampus and parahippocampal gyrus showed greater connectivity in the CM group than in the non-CM group while controlling for adaptive functioning (Figure 2; Table 2). Other regions previously identified as showing group differences in connectivity were no longer significant when adaptive functioning was included in the statistical model.
Figure 2.
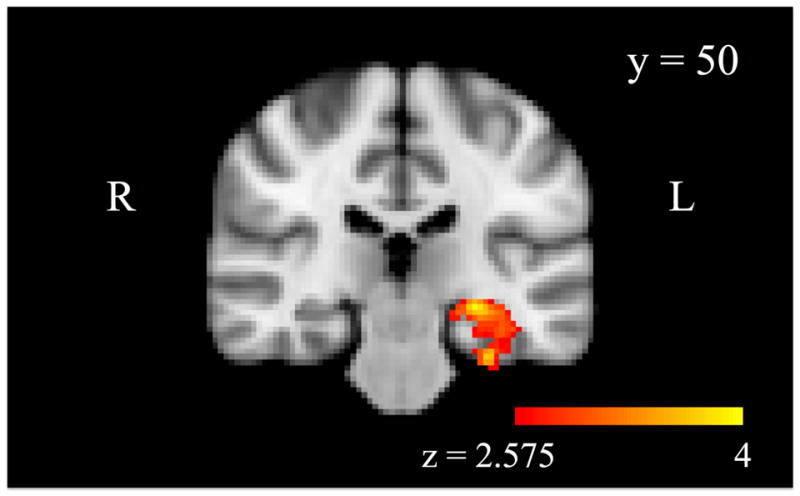
A region of the left hippocampus shows task-related (emotion-matching > shape-matching) functional connectivity with the bilateral amygdala (psychophysiological interaction; PPI) (emotion-matching > shape-matching) that is significantly greater for the childhood maltreatment group than the comparison group, when controlling for adult adaptive functioning, p < .05 cluster corrected.
Table 2.
Maltreatment and adult adaptive functioning effects on bilateral amygdala functional connectivity as revealed through the psychophysiological interaction (PPI) analysis
| MNI Coordinates
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Region | Side | Volume (mm3) | z Max | x | y | z | z mean | z standard deviation |
| Maltreatment Effect (Maltreated > Comparison) Controlling for Adaptive Functioning | ||||||||
|
| ||||||||
| Hippocampus | L | 3816 | 4.10 | −36 | −22 | −26 | 2.985 | 0.313 |
|
| ||||||||
| Adaptive Functioning Effect (Developmental Task Score), Controlling for Maltreatment Group | ||||||||
|
| ||||||||
| Cingulate Gyrus | L/R | 8016 | 4.37 | −6 | −6 | 28 | 2.922 | 0.310 |
| DMPFC | L/R | 7600 | 4.34 | 8 | 28 | 46 | 3.004 | 0.315 |
| Parietal Cortex | R | 5528 | 4.10 | 18 | −56 | 46 | 2.955 | 0.315 |
| Parietal Cortex | L | 5232 | 4.18 | −46 | −52 | 40 | 2.909 | 0.261 |
Note: All analyses included sex, age, and accuracy on emotion-matching trials as demeaned covariates. DMPFC = dorsomedial prefrontal cortex
PPI Effects: Effect of Adaptive Functioning, Controlling for Group
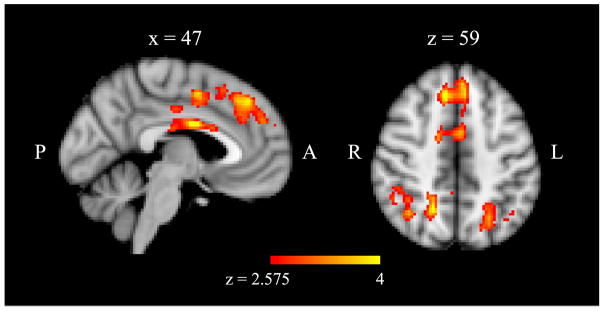
Developmental task score was a significant predictor of FC (stronger amygdala connectivity during emotion matching than shape matching) in several regions while controlling for maltreatment status (Figure 3, Table 2). PPI signal in the cingulate gyrus, dorsomedial PFC and in the bilateral parietal cortex including portions of the lateral occipital, angular gyrus, and supramarginal gyri was negatively associated with adaptive functioning. No regions showed a positive correlation with resilience.
Figure 3.
Regions in which task-related (emotion-matching > shape-matching) functional connectivity with the bilateral amygdala (psychophysiological interaction; PPI) correlated with adult adaptive functioning, controlling for maltreatment status, p < .05 cluster corrected.
PPI Effects: Maltreatment x Adaptive Functioning Interactions
The interaction of maltreatment with adaptive functioning did not predict amygdala- based FC in any brain region after cluster correction.
Discussion
This study examined the role of adaptive functioning in modulating emotion-related frontolimbic FC in adults with and without a history of CM. As such, it makes several important contributions to the existing maltreatment literature. First, unlike many adult studies of maltreatment, this sample includes a comparison group matched on socioeconomic and risk status. Furthermore, given that adaptive functioning did not differ between groups in adulthood, we were better able to isolate specific effects of maltreatment without confounding group differences in poverty, general risk, or adult functioning (43). This is particularly important given the widespread and pervasive effects of poverty on development (44–46), brain structure (47), and amygdala and frontal lobe activity in response to emotion laden stimuli (48, 49). Second, the sample was assessed prospectively and therefore typical measurement error issues of retrospective report were avoided (50). Third, this study considered adult adaptive functioning in the context of development in a high-risk environment using a multidimensional composite measure. This allowed for greater understanding of the interactions between CM and resilient functioning in adulthood than dichotomous measures, such as the presence of psychiatric diagnosis, which fails to account for subthreshold or transient symptomatology and other functional disturbances (51). Finally, the current study is unique in its focus on altered brain networks involved in emotion processing. By comparing FC during the emotion-laden and comparison conditions using PPI, we were able to determine the specific effects of CM and adaptive functioning on connectivity in the context of emotion.
The PPI results were consistent with convergent findings of increased emotion-based frontolimbic connectivity in both adult PTSD (52–54) and CM samples (20, 21, 55). Specifically, the current study found greater amygdala-based connectivity in the CM group than in the non-CM group within the dorsomedial- and right dorsolateral PFC. Further, we expected childhood maltreatment status to interact with adult functioning to predict emotion-related amygdala connectivity, such that adaptive functioning would have the strongest effect in the maltreated group. However, no such interaction effects were found. Instead, results emphasize the importance of positive adaptation following early adversity in this high-risk sample, regardless of maltreatment history. Specifically, the dorsomedial PFC region that showed a difference in emotion-related amygdala connectivity by group was no longer significant when variance due to adaptive functioning was also included in the model. Additional regions including more anterior regions of the dorsomedial PFC, the cingulate, and bilateral parietal regions also showed emotion-related connectivity with the amygdala that was related to adaptive functioning. Therefore, our findings suggest that CM does not relate to frontolimbic PPI connectivity directly when adaptive functioning is considered. This discrepancy emphasizes the importance of considering multiple levels of functioning when comparing groups.
Nevertheless, adaptive functioning does not account for all of the variance in amygdala-based connectivity between CM and non-CM groups. We found differences in emotion-related hippocampus-amygdala connectivity between the groups. Interactions between these structures are central to forming representations of emotional significance (56). Further, converging evidence suggests that the hippocampus and the amygdala may be especially vulnerable to chronic stress (57). We speculate that the differentially greater recruitment of the hippocampus-amygdala circuitry when processing emotional stimuli may reflect compensation for an inefficient network in the maltreated individuals. The group difference in PPI was observed even when controlling for adaptive functioning. Given that the comparison group in this study was well-matched on SES and demographic factors in childhood and thus also experienced chronic stressors associated with poverty, it seems likely that the observed group differences are specific to maltreatment history.
Taken together, these results suggest that neural processing of emotion differs between adults with and without histories of CM and also varies based on current functioning. Adult adaptive functioning, but not CM, exerted a unique effect on emotion-based frontolimbic connectivity even in groups matched on SES variables. Specifically, we found a relationship between adaptive functioning and task-related amygdala connectivity in the prefrontal region of the brain, including the medial PFC and cingulate gyrus. The results suggest that lower adaptive functioning is related to a greater difference in frontolimbic processing of emotional and non-emotional stimuli, regardless of maltreatment history. It is possible that frontolimbic circuitry in low-functioning individuals is inefficient and therefore must be strongly engaged when processing emotional stimuli. These prefrontal regions are thought to integrate multiple components of cognition and emotion, including motivational, evaluative, cognitive and emotional inputs (58). Converging research suggests that engagement of frontolimbic circuitry is necessary for cognitive regulation of emotion (59–63). The ability to process and respond appropriately to emotional stimuli, and to regulate one’s own emotional responses is likely critical to success in the everyday contexts represented in the adaptive functioning measure. Another potential factor of interest, adult internalizing symptoms, had negligible effects on the results (Supplement 1), and were not included in the primary analyses due to overlap with the adaptive functioning measure.
As previously noted, measures of adaptive functioning were also associated with emotion-related amygdala connectivity in more posterior parts of the brain, including areas in the parietal cortex that are implicated in attention processes (64). Interestingly, FC with the bilateral amygdala across the entire sample did not reveal connectivity differences between the emotion-matching and shape-matching conditions in these parietal regions. That is, the amygdala and parietal lobe did not show emotion-related amygdala connectivity on average in all participants, but PPI values in this region were significantly higher in individuals with higher adaptive functioning scores. It is possible that the emotion-based FC in the parietal lobe reflects compensatory mechanisms that individuals with high adaptive functioning employ while performing the face-matching task, such as enhanced attention.
Despite important contributions to the extant literature, this study had several limitations. While participants were followed longitudinally since childhood, MRI assessment was only conducted at the most recent time-point in adulthood. Therefore, we cannot determine the directionality of the relationships between maltreatment, adaptive functioning, and brain circuitry. Additionally, it is probable that current life stressors and factors related to adaptive functioning influenced which participants were able to continue in the study. For instance, some participants had employment conflicts prohibiting research participation, while others were unable to participate due to incarceration or health-related MRI contraindications (e.g., obesity or metal in body). Consequently, our sample represents a restricted range of developmental outcomes seen in high-risk, low-SES populations. While adaptive functioning is observed, sample characteristics (Table 1) indicate that this sample is not exceptionally resilient. It is possible that an even more robust effect of adaptive functioning on frontolimbic connectivity would be observed if the full range of developmental outcomes was included. We also do not know if the relationship between success on developmental tasks and amygdala connectivity would be the same in individuals from low-risk backgrounds. Further work with a low-risk comparison group is needed to determine if effects on frontolimbic connectivity are specific to adaptation following early adversity or if they can be generalized to adaptive functioning and competence, regardless of early environment. Replication with a larger sample is also warranted. Additionally, while adult adaptive functioning may indicate the capacity for resilience, further study of resilient processes throughout development is needed. Finally, a recent methods paper suggests use of more stringent clustering thresholds (65) and adoption of this conservative approach renders some of the reported regions non-significant (see Supplement 1).
In conclusion, we found that, when both maltreatment history and success on developmental tasks in adulthood were considered, only the latter was a significant predictor of frontolimbic connectivity during an emotion-matching task. This finding suggests that in high-risk populations, the ability to thrive despite setbacks is more related to frontolimbic efficiency during emotion processing than it is to maltreatment history. In contrast, hippocampus-amygdala connectivity during an emotion-matching task seems to be specific to maltreatment history, and not impacted by adaptive functioning. Maltreatment may have a significant impact on associative emotional processes, including memory, whereas adaptive functioning may be more strongly related to top-down cognitive control of emotion. Together, these findings suggest both that it is imperative to consider individual differences, such as adult adaptive functioning, when assessing group differences in maltreated samples, but also that maltreatment history has unique effects on neural circuitry within the limbic system above and beyond childhood poverty and current adaptive functioning. These results highlight the capacity for resilience despite CM and thereby support the utilization of intervention programs that promote positive adaptation.
Supplementary Material
Acknowledgments
This project was supported by funds from a McKnight Presidential Chair, William Harris Endowed Chair, and a Klaus J. Jacobs Research Prize (to D.C.), as well as imaging support from the Rochester Center for Brain Imaging and a pilot grant from the College of Arts, Science & Engineering, University of Rochester. Trainee support was provided by the University of Minnesota’s Institute of Child Development via a NIMH National Research Service Award T32-MH015755 (to L.A.D. and K.J.M.), and the Doris Duke Fellowship for the Promotion of Child Well-Being (to K.J.M.). We are grateful for support from the staff at the Mt. Hope Family Center, University of Rochester, and the Institute of Child Development at the University of Minnesota. The authors acknowledge the Minnesota Supercomputing Institute (MSI) at the University of Minnesota for providing resources that contributed to the research results reported within this paper. We thank Emily Hunt and Pat Weber for data collection. Many thanks to the families and longitudinal participants of the Mt. Hope Family Center for their participation.
Footnotes
Financial Disclosures The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Cicchetti D, Toth SL. Child maltreatment. Annu Rev Clin Psychol. 2005;1:409–438. doi: 10.1146/annurev.clinpsy.1.102803.144029. [DOI] [PubMed] [Google Scholar]
- 2.Maughan A, Cicchetti D. Impact of child maltreatment and interadult violence on children’s emotion regulation abilities and socioemotional adjustment. Child Dev. 2002;73:1525–1542. doi: 10.1111/1467-8624.00488. [DOI] [PubMed] [Google Scholar]
- 3.Pollak SD, Cicchetti D, Hornung K, Reed A. Recognizing emotion in faces: developmental effects of child abuse and neglect. Dev Psychol. 2000;36:679–688. doi: 10.1037/0012-1649.36.5.679. [DOI] [PubMed] [Google Scholar]
- 4.Pollak SD, Tolley-Schell SA. Selective attention to facial emotion in physically abused children. J Abnorm Psychol. 2003;112:323–338. doi: 10.1037/0021-843x.112.3.323. [DOI] [PubMed] [Google Scholar]
- 5.Young JC, Widom CS. Long-term effects of child abuse and neglect on emotion processing in adulthood. Child Abuse Negl. 2014;38:1369–1381. doi: 10.1016/j.chiabu.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rellini AH, Vujanovic AA, Gilbert M, Zvolensky MJ. Childhood maltreatment and difficulties in emotion regulation: Associations with sexual and relationship satisfaction among young adult women. Journal of Sex Research. 2012;49:434–442. doi: 10.1080/00224499.2011.565430. [DOI] [PubMed] [Google Scholar]
- 7.Spertus IL, Yehuda R, Wong CM, Halligan S, Seremetis SV. Childhood emotional abuse and neglect as predictors of psychological and physical symptoms in women presenting to a primary care practice. Child Abuse Negl. 2003;27:1247–1258. doi: 10.1016/j.chiabu.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Egeland B. Taking stock: childhood emotional maltreatment and developmental psychopathology. Child Abuse Negl. 2009;33:22–6. doi: 10.1016/j.chiabu.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert R, Widom CS, Browne K, Fergusson D, Webb E, Janson S. Burden and consequences of child maltreatment in high-income countries. Lancet. 2009;373:68–81. doi: 10.1016/S0140-6736(08)61706-7. [DOI] [PubMed] [Google Scholar]
- 10.van Harmelen AL, van Tol MJ, Van DerWee NJA, Veltman DJ, Aleman A, Spinhoven P, et al. Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biol Psychiatry. 2010;68:832–838. doi: 10.1016/j.biopsych.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Cicchetti D, Toth SL, Maughan A. Handb Dev Psychopathol. 2. 2000. An ecological-transactional model of child maltreatment; pp. 689–722. [Google Scholar]
- 12.Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 13.van Harmelen AL, van Tol MJ, Demenescu LR, et al. Enhanced amygdala reactivity to emotional faces in adults reporting childhood emotional maltreatment. Soc Cogn Affect Neurosci. 2013;8:362–369. doi: 10.1093/scan/nss007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mueller SC, Maheu FS, Dozier M, Peloso E, Mandell D, Leibenluft E, Pine DS, Ernst M. Early life stress is associated with impairment in cognitive control in adolescence: an fMRI study. Neuropsychologia. 2010;48:3037–3044. doi: 10.1016/j.neuropsychologia.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elton A, Tripathi SP, Mletzko T, Young J, Cisler JM, James GA, et al. Childhood maltreatment is associated with a sex-dependent functional reorganization of a brain inhibitory control network. Human Brain Mapping. 2014;35:1654–1667. doi: 10.1002/hbm.22280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn Sci. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- 17.Phillips M, Ladouceur C, Drevets W. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex. 2011;21:1667–1673. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, et al. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc Natl Acad Sci. 2013;110:19119–19124. doi: 10.1073/pnas.1310766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fonzo GA, Flagan TM, Sullivan S, Allard CB, Grimes EM, Simmons AN, et al. Neural functional and structural correlates of childhood maltreatment in women with intimate-partner violence-related posttraumatic stress disorder. Psychiatry Res. 2013;211:93–103. doi: 10.1016/j.pscychresns.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jedd K, Hunt RH, Cicchetti D, Hunt E, Cowell RA, Rogosch FA, et al. Long-term consequences of childhood maltreatment: Altered amygdala functional connectivity. Dev Psychopathol. 2015;27:1577–1589. doi: 10.1017/S0954579415000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luthar SS, Cicchetti D, Becker B. The Construct of Resilience: A Critical Evaluation and Guidelines for Future Work. Child Dev. 2000;71:542–562. doi: 10.1111/1467-8624.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garmezy N. Stress-resistant children: The search for protective factors. In: Stevenson J, editor. Recent research in developmental psychopathology. Oxford: Pergamon Press; 1985. pp. 213–233. [Google Scholar]
- 24.Cicchetti D, Rogosch FA, Lynch M, Holt KD. Resilience in maltreated children: Processes leading to adaptive outcome. Dev Psychopath. 1993;5:629. [Google Scholar]
- 25.New AS, Fan J, Murrough JW, Liu X, Liebman RE, Guise KG, et al. A Functional Magnetic Resonance Imaging Study of Deliberate Emotion Regulation in Resilience and Posttraumatic Stress Disorder. Biol Psychiatry. 2009;66:656–664. doi: 10.1016/j.biopsych.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 26.van der Werff SJA, Pannekoek JN, Veer IM, van Tol MJ, Aleman A, Veltman DJ, et al. Resilience to childhood maltreatment is associated with increased resting-state functional connectivity of the salience network with the lingual gyrus. Child Abus Negl. 2013;37:1021–1029. doi: 10.1016/j.chiabu.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Masten A, Tellegen A. Resilience in developmental psychopathology: Contributions of the Project Competence Longitudinal Study. Dev Psychopathol. 2012;24:345–361. doi: 10.1017/S095457941200003X. [DOI] [PubMed] [Google Scholar]
- 28.Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: Effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- 29.Herringa RJ, Burghy CA, Stodola DE, Fox ME, Davidson RJ, Essex MJ. Enhanced Prefrontal-Amygdala Connectivity Following Childhood Adversity as a Protective Mechanism Against Internalizing in Adolescence. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:326–334. doi: 10.1016/j.bpsc.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogosch FA, Oshri A, Cicchetti D. From child maltreatment to adolescent cannabis abuse and dependence: a developmental cascade model. Dev Psychopathol. 2010;22:883–897. doi: 10.1017/S0954579410000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cicchetti D, Toth SL, Manly JT. Unpublished manuscript. Mt. Hope Family Center; Rochester, NY: 2003. Maternal maltreatment classification interview. [Google Scholar]
- 32.Achenbach TM, Rescorla LA. Manual for ASEBA adult forms and profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2003. [Google Scholar]
- 33.Beck AT, Steer RA, Brown GK. Beck depression inventory-II. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 34.Cicchetti D, Cohen DJ. Perspectives on developmental psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology. Vol. 1. New York: Wiley; 1995. pp. 13–20. [Google Scholar]
- 35.Masten AS, Cicchetti D. Resilience in development: Progress and transformation. Developmental psychopathology. 2016;4:1–63. [Google Scholar]
- 36.Havighurst RJ. Research on the developmental-task concept. The School Review. 1956;64:215–223. [Google Scholar]
- 37.Schulenberg JE, Bryant AL, O’Malley PM. Taking hold of some kind of life: How developmental tasks relate to trajectories of well-being during the transition to adulthood. Dev Psychopathol. 2004;16:1119–1140. doi: 10.1017/s0954579404040167. [DOI] [PubMed] [Google Scholar]
- 38.Ekman P, Friesen WV. Facial action coding system: A technique for the measurement of facial movement. Palo Alto, CA: Consulting Psychologists Press; 1978. [Google Scholar]
- 39.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 40.O’Reilly JX, Woolrich MW, Behrens TE, Smith SM, Johansen-Berg H. Tools of the trade: Psychophysiological interactions and functional connectivity. Soc Cogn Affect Neurosci. 2012;7:604–609. doi: 10.1093/scan/nss055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 42.Fakra E, Salgado-Pineda P, Delaveau P, Hariri AR, Blin O. Neural bases of different cognitive strategies for facial affect processing in schizophrenia. Schizophr Res. 2008;100:191–205. doi: 10.1016/j.schres.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 43.Hussey JM, Chang JJ, Kotch JB. Child maltreatment in the United States: prevalence, risk factors, and adolescent health consequences. Pediatrics. 2006;118:933–942. doi: 10.1542/peds.2005-2452. [DOI] [PubMed] [Google Scholar]
- 44.Brooks-Gunn J, Duncan GJ. The Effects of Poverty on Children. The Future of Children. 1997;7:55–71. [PubMed] [Google Scholar]
- 45.Leventhal T, Brooks-Gunn J. The neighborhoods they live in: The effects of neighborhood residence on child and adolescent outcomes. Psychol Bull. 2000;126:309–337. doi: 10.1037/0033-2909.126.2.309. [DOI] [PubMed] [Google Scholar]
- 46.Costello EJ, Compton SN, Keeler G, Angold A. Relationships Between Poverty Psychopathology. A Natural Experiment. JAMA. 2003;290(15):2023–2029. doi: 10.1001/jama.290.15.2023. [DOI] [PubMed] [Google Scholar]
- 47.Hanson JL, Hair N, Shen DG, Shi F, Gilmore JH, Wolfe BL, Pollak SD. Family poverty affects the rate of human infant brain growth. PLoS ONE. 2013:8. doi: 10.1371/journal.pone.0080954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim P, Evans GW, Angstadt M, Ho SS, Sripada CS, Swain JE, et al. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proc Natl Acad Sci. 2013;110:18442–18447. doi: 10.1073/pnas.1308240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Javanbakht A, King AP, Evans GW, Swain JE, Angstadt M, Phan KL, et al. Childhood Poverty Predicts Adult Amygdala and Frontal Activity and Connectivity in Response to Emotional Faces. Front Behav Neurosci. 2015;9:154. doi: 10.3389/fnbeh.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hardt J, Rutter M. Validity of adult retrospective reports of adverse childhood experiences: Review of the evidence. J Child Psychol Psychiatry. 2004;45:260–273. doi: 10.1111/j.1469-7610.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- 51.Bonanno GA. Loss, Trauma, and Human Resilience: Have we underestimated the human capacity to thrive after extremely adverse events? Am Psychol. 2004;59:20–28. doi: 10.1037/0003-066X.59.1.20. [DOI] [PubMed] [Google Scholar]
- 52.Dunkley BT, Pang EW, Sedge PA, Jetly R, Doesburg SM, Taylor MJ. Threatening faces induce fear circuitry hypersynchrony in soldiers with post-traumatic stress disorder. Heliyon. 2016;2:e00063. doi: 10.1016/j.heliyon.2015.e00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gilboa A, Shalev AY, Laor L, Lester H, Louzoun Y, Chisin R, Bonne O. Functional connectivity of the prefrontal cortex and the amygdala in posttraumatic stress disorder. Biol Psychiatry. 2004;55:263–272. doi: 10.1016/j.biopsych.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 54.Jacques PLS, Botzung A, Miles A, Rubin DC. Functional neuroimaging of emotionally intense autobiographical memories in post-traumatic stress disorder. J Psychiatr Res. 2011;45:630–637. doi: 10.1016/j.jpsychires.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, Lieberman MD. Neural responses to emotional stimuli are associated with childhood family stress. Biol Psychiatry. 2006;60:296–301. doi: 10.1016/j.biopsych.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 56.Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 57.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 58.Bush G, Luu, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in cognitive sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 59.Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala–frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry. 2010;167:545–554. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, … Tottenham N. A Developmental Shift from Positive to Negative Connectivity in Human Amygdala – Prefrontal Circuitry. The Journal of Neuroscience. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, et al. A Developmental Shift from Positive to Negative Connectivity in Human Amygdala – Prefrontal Circuitry. J Neurosci. 2013;33:4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological Substrates of Emotional Reactivity and Regulation in Adolescence During an Emotional Go-Nogo Task. Biol Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 65.Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci. 2016 doi: 10.1073/pnas.1602413113. 201602413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.