Supplemental Digital Content is available in the text
Keywords: case-parent trio, food allergy, maternal genetic effect, multinomial likelihood ratio tests, parent-of-origin effect
Abstract
Previous genetic studies of food allergy (FA) have mainly focused on inherited genotypic effects. The role of parental genotypic effects remains largely unexplored. Leveraging existing genome-wide association study (GWAS) data generated from the Chicago Food Allergy Study, we examined maternal genotypic and parent-of-origin (PO) effects using multinomial likelihood ratio tests in 588 complete and incomplete Caucasian FA trios. We identified 1 single nucleotide polymorphism with significant (P < 5×10−8) maternal effect on any FA (rs4235235), which is located in a noncoding RNA (LOC101927947) with unknown function. We also identified 3 suggestive (P < 5×10−7) loci with maternal genetic effects: 1 for any FA (rs976078, in a gene desert region on 13q31.1) and 2 for egg allergy (rs1343795 and rs4572450, in the ZNF652 gene, where genetic variants have been associated with atopic dermatitis). Three suggestive loci with PO effect were observed: 1 for peanut allergy (rs4896888 in the ADGB gene) and 2 for any FA in boys only (rs1036504 and rs2917750 in the IQCE gene). Findings from this family-based GWAS of FA provided some preliminary evidence on maternal genotypic or PO effects on FA. Additional family-based studies are needed to confirm our findings and gain new insight into maternal and paternal genetic contribution to FA.
1. Introduction
Food allergy (FA), an immunoglobulin (Ig) E-mediated hypersensitivity reaction to food, affects approximately 5% to 8% of US children[1,2] and is an important clinical and public health problem worldwide.[3,4] Our current understanding of the causes and underlying biological mechanisms of FA remains limited.
Previous familial aggregation[5] and twin[6–8] studies have demonstrated that FA is under strong genetic control with heritability estimates ranging from 15% to 82%. To date, few genetic loci for FA have been consistently identified and replicated, and there are only 2 published genome-wide association studies (GWAS) of FA.[9,10] Genetic variants in HLA Class II genes have been significantly associated with peanut allergy (PA) in several candidate genes[11–15] and a GWAS[9], while the STAT6,[16]CD14,[17] and FLG[18] genes associated with PA have mainly been reported in small single-gene studies. As such, similar to other complex traits, “missing heritability” remains is a major challenge in dissecting the genetic basis of FA. It should be noted that all previous genetic studies were primarily focused on individual genotypes without considering maternal influence during pregnancy and parental origins. As an early onset disorder, maternal genotypes may play crucial roles in the development of FA, possibly through their influence on the intrauterine environment. Furthermore, parent-of-origin (PO) effects, where the effect of an inherited allele depends on being maternally- or paternally transmitted, may also conceivably underlie the missing heritability. This type of research is critically needed using a family-based study design.
This study is a natural extension of our published GWAS of FA in FA families from the Chicago Food Allergy Study cohort, which aimed to identify offspring inherited susceptibility variants for FA.[9] To extend our early findings, this study sought to test for maternal genetic effects and PO effects on FA, by leveraging the existing GWAS data from the trios (FA affected kid and his/her parents).
2. Methods
2.1. Study participants
2.1.1. The Chicago Food Allergy Study
This study included 588 (482 complete and 106 incomplete) Caucasian case-parent trios, a subset of participants in the Chicago Food Allergy Study cohort. A detailed description of the GWAS of FA previously conducted in this cohort has been published.[9] Briefly, a total of 1315 children and 1444 biological parents were genotyped using the Illumina HumanOmni-Quad Beadchip. We excluded 65 participants whose data failed quality control (QC) and 497 individuals of non-European ancestry, as detailed in Hong et al.[9] Among the remaining 2197 individuals of European ancestry, we selected 1 FA affected child from each family by first considering the index child and then the first enrolled sibling only if the genotype information for the FA index child was unavailable. FA was defined in Hong et al and is also detailed below. A total of 482 complete trios with any FA, 19 father-only FA child pairs, 59 mother-only FA child pairs, and 28 singleton FA children were analyzed in the present study. The detailed study design is shown in Figure 1. This study was approved by the Institutional Review Board (IRB) of Ann & Robert Lurie Children's Hospital of Chicago and the IRB of Johns Hopkins Bloomberg School of Public Health.
Figure 1.
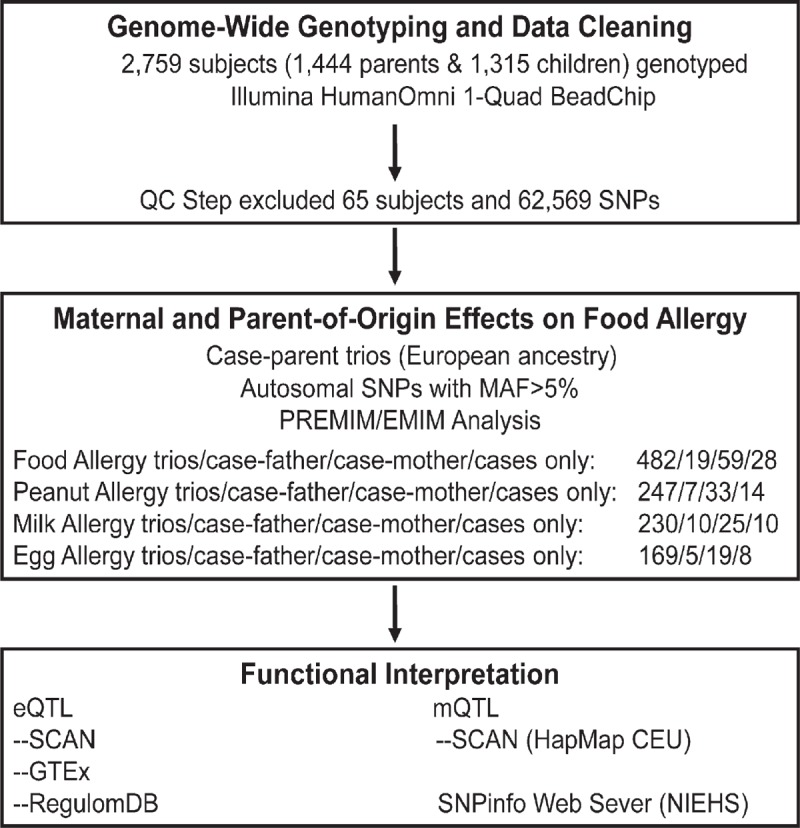
Workflow diagram of the study.
We defined FA using the same criteria as previously published[9]: self-report of a clear clinical allergic reaction after ingestion of a specified food (e.g., peanut, egg, cow's milk, soy, wheat, walnut, fish, shellfish, and/or sesame seed); and sensitization to the same specified food with a detectable food-specific IgE (≥ 0.10 kU/L) and/or a positive skin prick test (SPT) to the specified food (e.g., mean wheal diameter 3 mm or greater than the saline control). “Any FA” was defined as a child having FA to any of the nine common food allergens as defined above.
2.2. Statistical methods
2.2.1. Maternal and parent-of-origin effects
We analyzed the maternal genetic effects and parent-of-origin (PO) effects for any FA and 3 common types of FA (peanut, egg white, and cow's milk) using estimation of maternal, imprinting and interaction effects using multinomial modelling (EMIM), a tool that directly maximizes multinomial likelihood by utilizing complete and incomplete trios.[19,20] EMIM has been recommended for testing maternal and PO effects because of its consistent type I error rate, generally strong power, and flexible implementation over several other family-based association tests of maternal and PO effects, such as log-linear models in the LEM software[21] and conditional on parental genotypes and others.[22] We estimated maternal genetic effects by comparing the likelihood of 2 models: A full model with 2 offspring genotypic risk parameters and one maternal genotypic risk factor (under an additive genetic model for the minor allele of each SNP); and a null model with 2 offspring genotypic risk parameters only. It should be noted that, to ensure the validity of the likelihood ratio test, we allowed 2 offspring genotypic risk parameters to saturate the offspring effects. Similarly, we estimated PO effects by comparing a full model with 2 offspring and 2 maternal genotypic risk parameters, and a PO effect parameter with a null model of having the 4 genotypic parameters only. We declared an SNP with genome-wide significance based on a conventional P-value of 5×10–8; while suggestive loci were based on a P-value of less than 5×10–7. Given that boys are more susceptible to FA than girls (boy/girl ratio = 1.8),[23] we explored maternal genetic effects and PO effects stratified by the gender of the FA-affected child.
2.2.2. Gene-based association test
GATEs is an extended Simes procedure to obtain a gene-level statistical significance by combining multiple SNP P-values from the single-marker association test while accounting for the correlations among SNPs based on their linkage disequilibrium (LD) patterns. Using the Knowledge-Based Mining System for Genome-Wide Genetic Studies (KGG),[24] we first assigned SNPs within 10 kb of the gene boundary on either side, and then applied the GATEs methods for gene-based association tests using SNP P-values from EMIM. LD structures between SNPs were based on 1000G Phase I integrated release haplotypes (v3) (http://www.sph.umich.edu/csg/abecasis/MACH/download/1000G.2012–03–14.html).
2.2.3. Bioinformatics and public databases
We used the following bioinformatics and public databases to help understand the potential function of the SNPs identified from the tests: SNP Annotation and Proxy Search (SNAP) to search for proxy SNPs (high LD measures, r2>0.8) of the top hits from the 1000 Genome Pilot 1 database; LDlink to search for proxy SNPs of the top hits from the 1000 Genome Project Phase 3 database; SNP and CNV Annotation Database (SCAN)[25] and genotype-tissue expression (GTEx) to determine if an SNP is associated with gene expression (eQTL) across different tissues; RegulomDB to comprehensively understand the potential regulatory function of an SNP via its annotation to the known or predicted regulatory elements (e.g., DNAase hypersensitivity, transcription factors binding sites, etc.); and SNPinfo Web Server to predict an SNP function.
3. Results
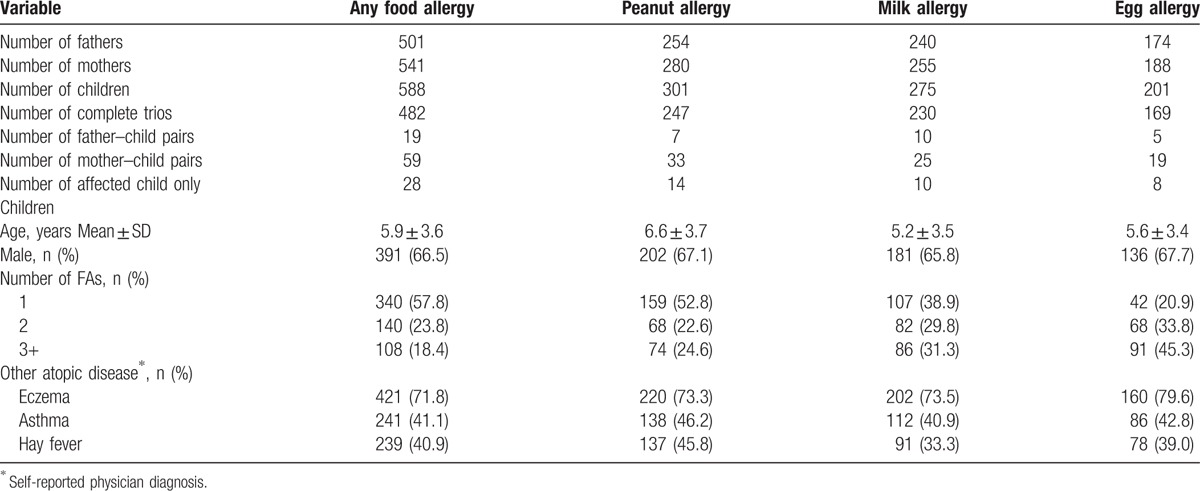
Table 1 lists the demographic and clinical characteristics of the study participants. The numbers of children affected with any FA, peanut allergy, milk allergy, and egg allergy were 588, 301, 275, and 201, respectively. The mean age of these FA children was around 6 years old, and over 65% were boys. A majority of them had one or more self-reported allergic diseases, such as eczema, asthma and hay fever.
Table 1.
Demographic characteristics of the Caucasian participants selected from the Chicago Food Allergy Study in the genome-wide study of maternal and parent-of-origin effects on food allergy.
3.1. Maternal genetic effects
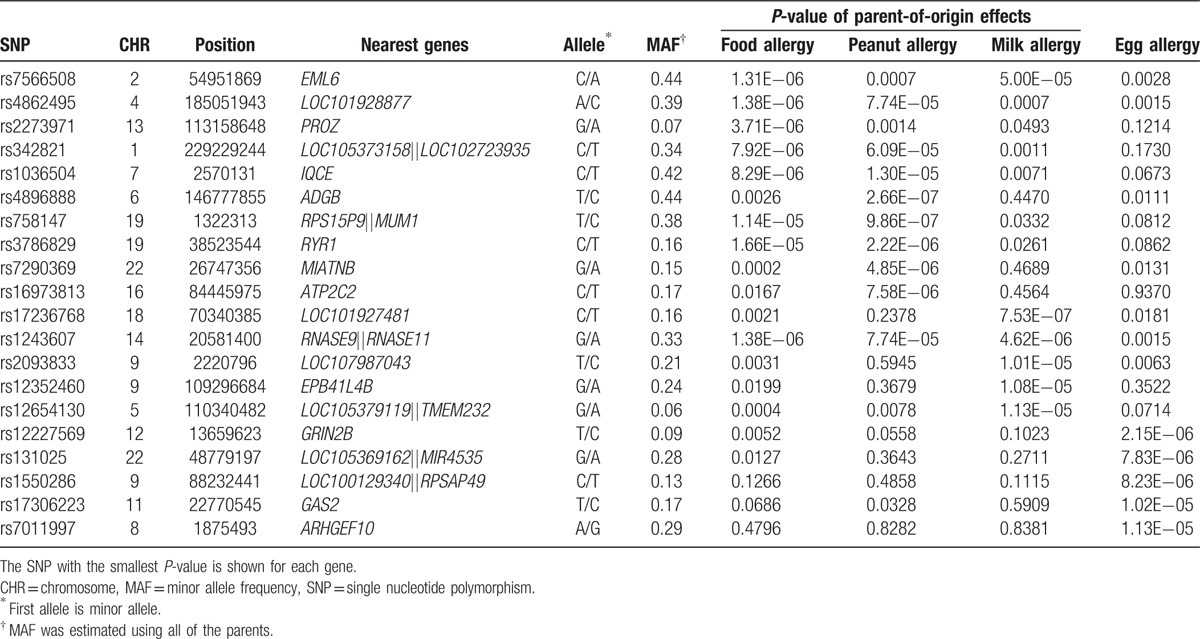
The maternal genetic effects for any FA, peanut allergy, egg allergy, and milk allergy are shown in the Manhattan plots (Fig. 2). We summarized maternal genetic effects by listing the top 5 loci identified from each of the 4 tested phenotypes (Table 2), and presenting the SNPs with a P-value of < 10–5 (Supplementary Table 1). The estimated relative risk (RR) for any FA ranged from 0.36 to 0.66 and from 1.94 to 3.37 for having one minor allele. We also summarized gene-based tests of maternal genetic effects by presenting the top 5 genes for each phenotype (Supplementary Table 2) and gender-stratified results for any FA (Supplementary Fig. 1).
Figure 2.
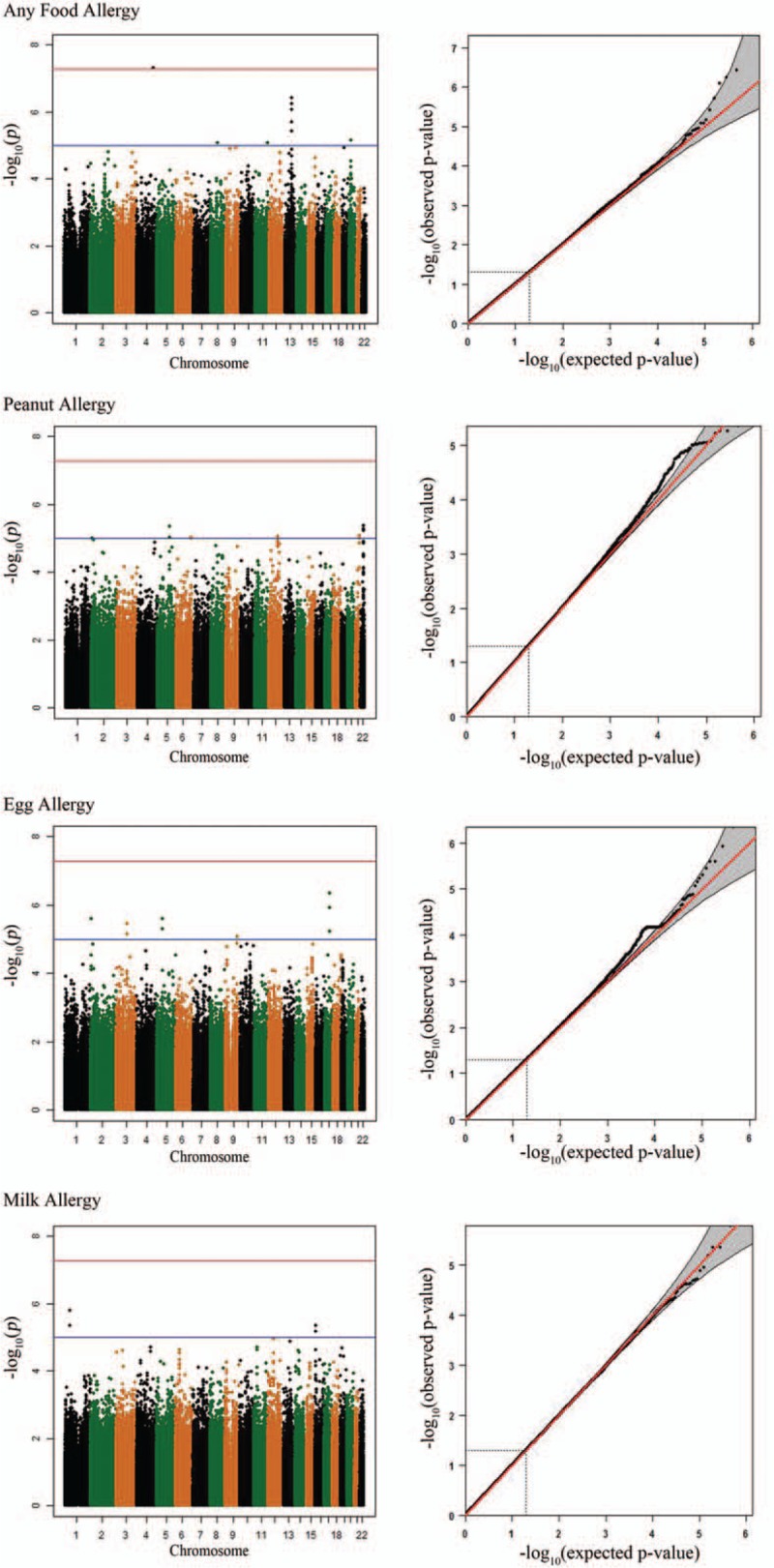
Manhattan plots and Q–Q plots for maternal effects on food allergy.
Table 2.
Top 5 loci identified in the tests of maternal genetic effects on any food allergy, peanut, milk and egg allergy in 588 complete and incomplete trios of European ancestry.
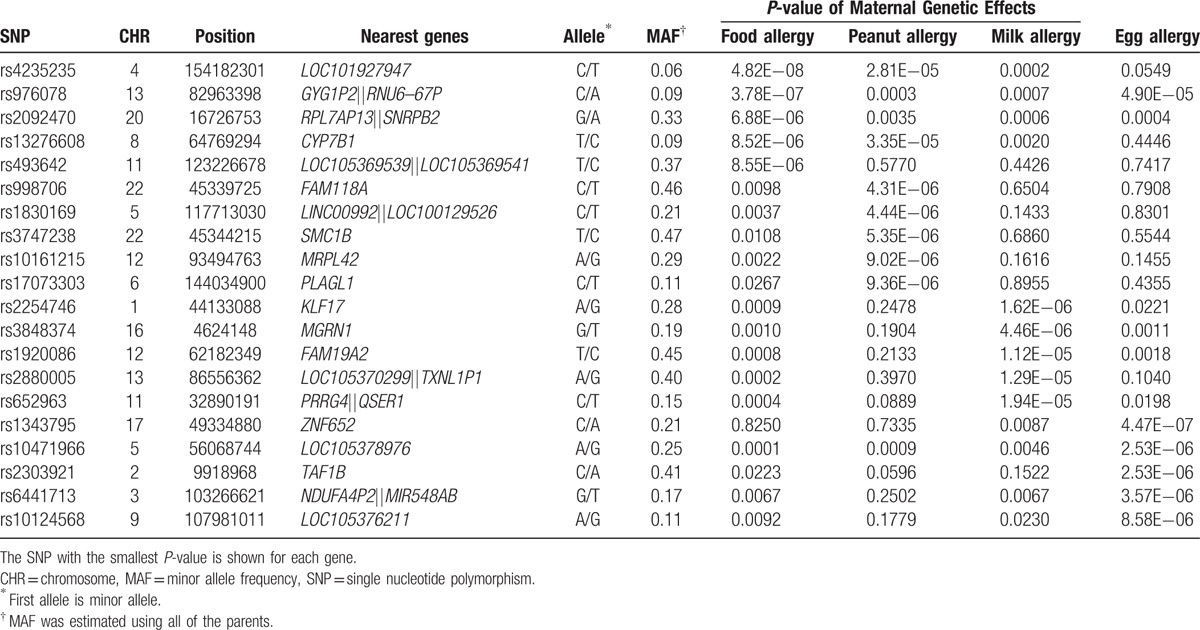
Only one SNP, rs4235235 on chromosome 4, reached the genome-wide significance level (P = 4.82×10–8) for any FA, which was not supported by any evidence from nearby SNPs. No other SNPs were in high LD (r2>0.8) with this SNP within 500 kb in a European population (CEU) according to 2 bioinformatics browsers: SNAP and LDlink. rs4235235 is annotated to a non-coding RNA (LOC101927947) with no known function, and there is no evidence to support this SNP's functional role according to several of the bioinformatics databases listed in the Methods.
We identified 2 suggestive loci annotated by SNPs with P-values <5×10–7. One was rs976078 on chromosome 13q31.1 (P = 3.78×10–7) for any FA, and the others were rs1343795 and rs4572450 (P = 4.47×10–7) on chromosome 17 for egg allergy. The corresponding Q–Q plots show some deviations from the diagonal lines at the end of the scale (Fig. 2). rs976078 is located in a gene desert region on 13q31.1. Two intronic SNPs (rs4572450 and rs1343795) suggestively associated with egg allergy are located in gene ZNF652 for which genetic variants have been associated with atopic dermatitis.
No statistically significant maternal genetic effects on FA or any specific FA were identified when we re-analyzed data among any FA affected boys (N = 391) only or any FA affected girls (N = 197) only. In addition, we did not observe any statistically significant maternal-fetal genetic interaction effects on any FA (data not shown).
3.2. PO effects
Among the same study participants, we examined the PO effects on the 4 FA phenotypes. From the Manhattan and Q–Q plots (Fig. 3), we found that no SNP reached the genome-wide significance level. One suggestive PO effect on peanut allergy at rs4896888 in the androglobin (ADGB) gene was observed. Similar to what was done for maternal effects, we summarized the results into the top 5 loci for each trait (Table 3), SNPs with P < 10–5 (Supplementary Table 3) and the top 5 genes from gene-based PO effects (Supplementary Table 4). When the data were stratified by the gender of the FA-affected children, rs1036504 and rs2917750 in gene IQ motif containing E (IQCE) reached the suggestive threshold of 5×10–7 for any FA in boys (Supplementary Fig. 2). These 3 SNPs have no potential function according to the bioinformatics databases. Neither ADGB nor IQCE is imprinted or predicted to be an imprinted gene in human beings according to the Genomic Imprinting database (http://www.geneimprint.com/site/home).
Figure 3.
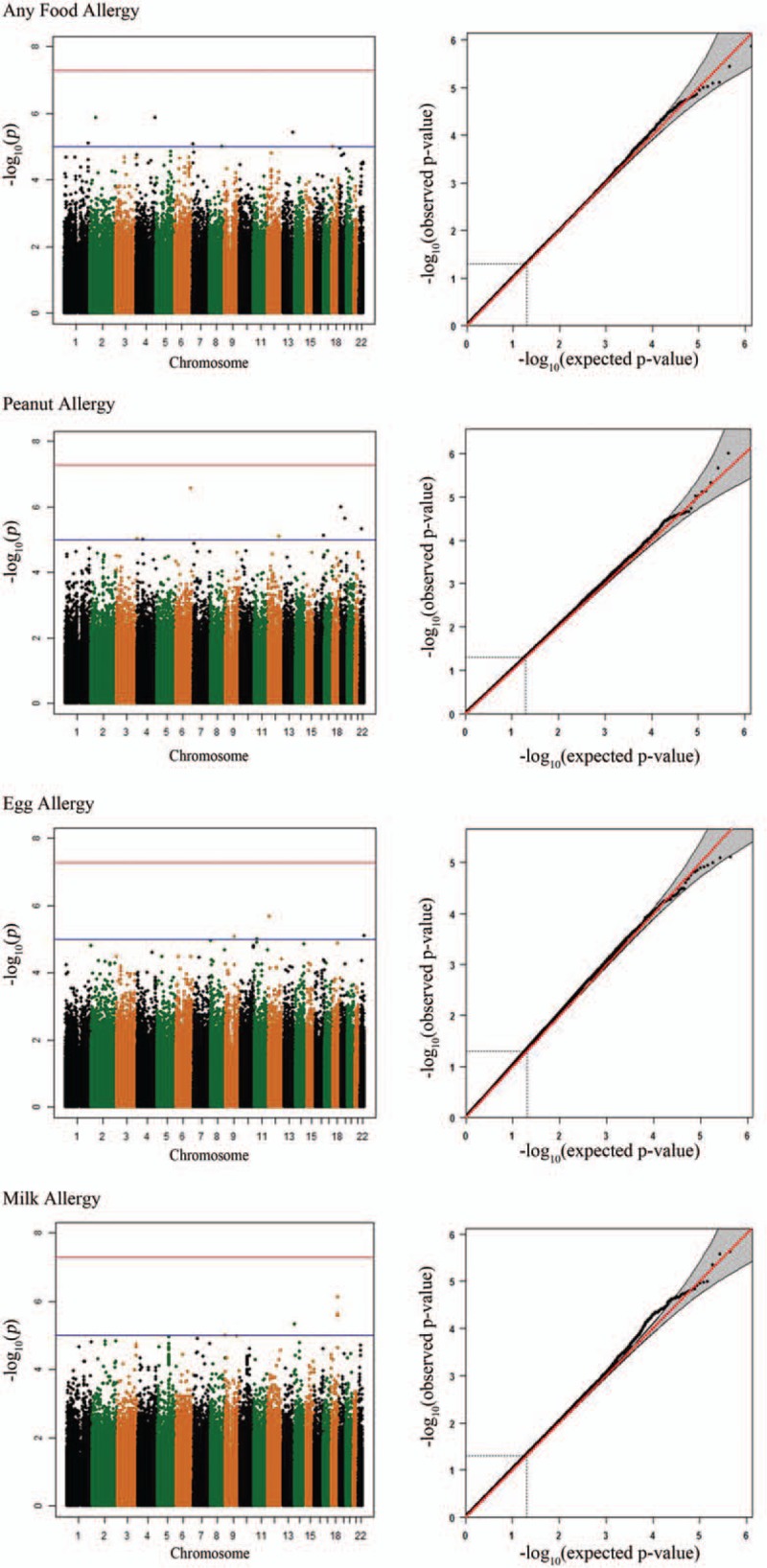
Manhattan plots and Q–Q plots for parent-of-origin effects on food allergy.
Table 3.
Top 5 loci identified in the tests of parent-of-origin effects on any food allergy, peanut, milk, and egg allergy in 588 complete and incomplete trios of European ancestry.
4. Discussion
As an early onset disorder, FA is likely to be determined not only by offspring risk factors but also by parental risk factors, particularly those related to maternal exposures during the pregnancy (in-utero environment) and breastfeeding period. The family study design offers the advantage of direct testing for the effects of fetal and maternal genotypes and allows for the separation of these effects from the parental origin in a robust manner. Based on the published literature to date, our GWAS of FA in the Chicago Food Allergy Study cohort was the first and is still the only FA study to use a family-based study design. As such, it is worth presenting the findings for maternal genotypic effects and PO effects on FA at a genome-wide level in this well-phenotyped cohort of children. Findings from ∼600 Caucasian FA trios provided some preliminary evidence for maternal genotypic effects or PO effects on the risk of FA in the offspring. Top hits based on loose criteria defined in Section 2 (e.g., P < 5×10–7) suggest that maternal metabolic alterations that occur pre- and peri-natally might increase the risk of any FA as well as peanut allergy in the offspring. Our findings also suggest that maternal genetic susceptibility to atopic dermatitis might lead to a high incidence of egg allergy in the offspring.
rs4235235 and rs976078 are the top hits for maternal genotypic effects on any FA phenotype. rs4235235 is located in an unknown function ncRNA, and this SNP itself appears to have no potential function either. According to RegulomDB, rs976078 is located at the C/EBP motif and may affect distal C/EBP beta target gene expression through its influence on chromatin looping. rs976078 also may influence the structure or activity of its annotated lncRNA, which then regulates transcription by targeting transcription factors, such as C/EBP beta, to promoters of its target genes. In examining NCBI's Phenotype–Genotype Integrator, we found that rs976078's nearby SNPs (within 3MB) are enriched for GWAS significant signals (<5×10–8) for the following phenotypes: Follicle-Stimulating Hormone (rs10507928), C Reactive Protein (rs10507919), low-density lipoprotein cholesterol (rs10507949), type II diabetes (rs1359790) and intra-abdominal fat (rs1556775 and rs7328488). We suspect that 1 or 2 common genetic features in region13q31 could represent the underlying causal sequences for endocrine and metabolic outcomes, though none of the above mentioned genetic variants are in moderate or high LD with rs976078 and its nearby SNPs. It is possible that maternal metabolic alterations or their triggers that occurred during pregnancy (e.g., stress, weight gain) may interact with maternal genetic variants that influence the intrauterine environment or induce infant epigenetic variations from the prenatal period onwards, which in turn may increase FA risk in the offspring. The causal maternal genetic variants in this region will first need to be determined, and the nature of the underlying mechanisms will require further investigation using functional studies.
Other suggestive maternal genetic effects were observed on egg allergy. rs4572450 and its nearby SNPs are located in gene ZNF652, for which genetic variants were found to be significantly associated with blood pressure[26] and atopic dermatitis.[27] One of these previously reported GWAS hits, rs16948048, showed a marginally significant maternal genetic effect on egg allergy (RR = 1.38, 95%CI: 1.02–1.87, P = .03) in the present study. Findings from the HealthNuts study showed that maternal eczema was associated with a high risk of offspring egg allergy but not peanut allergy.[28] We suspect that maternal genetic variations in the ZNF652 gene may be associated with maternal eczema during pregnancy followed by the development of egg allergy in the offspring.
We only observed one suggestive locus with a PO effect on peanut allergy. This top hit has no evidence of support from nearby SNPs typed in the same Beadchip Array. According to the 1000 Genomes Project Database, SNPs that were un-typed but in high LD with rs4896888 in CEU are all located in the ADGB gene. When stratifying the samples by the gender of the FA-affected child, we found suggestive PO effects of IQCE genetic variants on any FA. Neither the ADGB nor the IQCE gene is known imprinted gene and neither appears to be regulated by any imprinted genes. Further exploration is warranted to identify whether allele-specific methylation of these 2 genes could explain the observed boy-specific PO effect on food allergy.
The notable limitations of our study were the small sample size in genome-wide tests and the lack of a replication study in an independent population. We had reasonable power to detect maternal effects. With 500 complete trios, when the RR for maternal effects was 2 the power was ≥0.90 for an SNP with an MAF between 0.2 and 0.5 at alpha = 1e−8. Our statistical power to detect imprinting effects of 2, however, was very limited (<1%). Maternal genetic variants with weak effects or exert their effects only through interaction with offspring's in utero environmental exposures cannot be detected with our current sample size. In contrast to several GWAS of common cancers, cardiovascular disease-related traits and even other allergic diseases (e.g., asthma, atopic dermatitis), the Chicago Food Allergy Study cohort was the first and is still the only FA study to use a family-based study design. We could not find another appropriate family-based, well-defined FA study to confirm our promising findings. Instead, we have presented the results and tentative interpretations based on the most current and best knowledge to inform future proof-of-concept studies. Finally, rather than exploring the effects of millions of un-typed SNPs, we searched for SNPs in high LD (R2>0.8) within 500 kb on each side of the top hits in the 1000 Genomes Project's database. No potentially functional proxy was identified for any of the suggestive SNPs, and thus we conclude that the imputed data could not be more informative than the genotyped data.
The significance of this study is that it represents the first attempt to explore maternal genotypic effects and PO effects on FA. While it is impractical to retrospectively obtain concise and timely measurements of various environmental exposures and epigenetic data before and at birth, the observed maternal genetic associations could be considered to be a proxy for the complex gene-environment interaction effects on the intrauterine environment and subsequently on fetal development. The family-based GWAS carried out here provides a unique opportunity to address the study questions. Additional family-based studies are needed to confirm our findings and gain new insight on early life origins of FA and offer new strategies for the prevention of FA.
Supplementary Material
Acknowledgments
We gratefully acknowledge the individuals and families who participated in the Chicago Food Allergy Study.
Footnotes
Abbreviations: FA = food allergy, GWAS = genome-wide association study, Ig = immunoglobulin, LD = linkage disequilibrium, MAF = minor allele frequency, PA = peanut allergy, PO = parent-of-origin, QC = quality control, RR = relative risk, SNP = single nucleotide polymorphism, SPT = skin prick test.
The study was supported in part by the Bunning Family and foundations and the National Institutes of Health (U01AI090727, R56AI080627, R03AI105294, R21AI079872). This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. KM was supported by Grant K23HL093302. HG and XT were supported by Grant R01DK064240. RPS was supported by Grants R37HL068546 and U19AI106683 from the NIH, and by the Ernest S. Bazley Foundation. XL also is supported by 100 Talents Program from the Chinese Academy of Sciences.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics 2011;128:e9–17. [DOI] [PubMed] [Google Scholar]
- [2].Sicherer SH, Sampson HA. 9 Food allergy. J Allergy Clin Immunol 2006;117(2 suppl Mini-Primer):S470–5. [DOI] [PubMed] [Google Scholar]
- [3].Gupta R, Sheikh A, Strachan DP, et al. Time trends in allergic disorders in the UK. Thorax 2007;62:91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sampson HA. Update on food allergy. J Allergy Clin Immunol 2004;113:805–19. quiz 820. [DOI] [PubMed] [Google Scholar]
- [5].Tsai HJ, Kumar R, Pongracic J, et al. Familial aggregation of food allergy and sensitization to food allergens: a family-based study. Clin Exp Allergy 2009;39:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hong X, Tsai HJ, Wang X. Genetics of food allergy. Curr Opin Pediatr 2009;21:770–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hourihane JO, Dean TP, Warner JO. Peanut allergy in relation to heredity, maternal diet, and other atopic diseases: results of a questionnaire survey, skin prick testing, and food challenges. BMJ 1996;313:518–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sicherer SH, Furlong TJ, Maes HH, et al. Genetics of peanut allergy: a twin study. J Allergy Clin Immunol 2000;106(1 pt 1):53–6. [DOI] [PubMed] [Google Scholar]
- [9].Hong X, Hao K, Ladd-Acosta C, et al. Genome-wide association study identifies peanut allergy-specific loci and evidence of epigenetic mediation in US children. Nat Commun 2015;6:6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Li J, Fung I, Glessner JT, et al. Copy number variations in CTNNA3 and RBFOX1 associate with pediatric food allergy. J Immunol 2015;195:1599–607. [DOI] [PubMed] [Google Scholar]
- [11].Boehncke WH, Loeliger C, Kuehnl P, et al. Identification of HLA-DR and -DQ alleles conferring susceptibility to pollen allergy and pollen associated food allergy. Clin Exp Allergy 1998;28:434–41. [DOI] [PubMed] [Google Scholar]
- [12].Dreskin SC, Tripputi MT, Aubrey MT, et al. Peanut-allergic subjects and their peanut-tolerant siblings have large differences in peanut-specific IgG that are independent of HLA class II. Clin Immunol 2010;137:366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Howell WM, Turner SJ, Hourihane JO, et al. HLA class II DRB1, DQB1 and DPB1 genotypic associations with peanut allergy: evidence from a family-based and case-control study. Clin Exp Allergy 1998;28:156–62. [DOI] [PubMed] [Google Scholar]
- [14].Madore AM, Vaillancourt VT, Asai Y, et al. HLA-DQB1∗02 and DQB1∗06:03P are associated with peanut allergy. Eur J Hum Genet 2013;21:1181–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shreffler WG, Charlop-Powers Z, Sicherer SH. Lack of association of HLA class II alleles with peanut allergy. Ann Allergy Asthma Immunol 2006;96:865–9. [DOI] [PubMed] [Google Scholar]
- [16].Amoli MM, Hand S, Hajeer AH, et al. Polymorphism in the STAT6 gene encodes risk for nut allergy. Genes Immun 2002;3:220–4. [DOI] [PubMed] [Google Scholar]
- [17].Dreskin SC, Ayars A, Jin Y, et al. Association of genetic variants of CD14 with peanut allergy and elevated IgE levels in peanut allergic individuals. Ann Allergy Asthma Immunol 2011;106:170–2. [DOI] [PubMed] [Google Scholar]
- [18].Brown SJ, Asai Y, Cordell HJ, et al. Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J Allergy Clin Immunol 2011;127:661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ainsworth HF, Unwin J, Jamison DL, et al. Investigation of maternal effects, maternal-fetal interactions and parent-of-origin effects (imprinting), using mothers and their offspring. Genet Epidemiol 2011;35:19–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Howey R, Cordell HJ. PREMIM and EMIM: tools for estimation of maternal, imprinting and interaction effects using multinomial modelling. BMC Bioinformatics 2012;13:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].van Den Oord EJ, Vermunt JK. Testing for linkage disequilibrium, maternal effects, and imprinting with (In)complete case-parent triads, by use of the computer program LEM. Am J Hum Genet 2000;66:335–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Connolly S, Heron EA. Review of statistical methodologies for the detection of parent-of-origin effects in family trio genome-wide association data with binary disease traits. Brief Bioinform 2015;16:429–48. [DOI] [PubMed] [Google Scholar]
- [23].Kelly C, Gangur V. Sex disparity in food allergy: evidence from the PubMed database. J Allergy (Cairo) 2009;2009:159845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li MX, Gui HS, Kwan JS, et al. GATES: a rapid and powerful gene-based association test using extended Simes procedure. Am J Hum Genet 2011;88:283–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang W, Gamazon ER, Zhang X, et al. SCAN database: facilitating integrative analyses of cytosine modification and expression QTL. Database (Oxford) 2015;2015:pii: bav025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Newton-Cheh C, Johnson T, Gateva V, et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet 2009;41:666–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ellinghaus D, Baurecht H, Esparza-Gordillo J, et al. High-density genotyping study identifies four new susceptibility loci for atopic dermatitis. Nat Genet 2013;45:808–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Koplin JJ, Allen KJ, Gurrin LC, et al. The impact of family history of allergy on risk of food allergy: a population-based study of infants. Int J Environ Res Public Health 2013;10:5364–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


