Fig. 4.
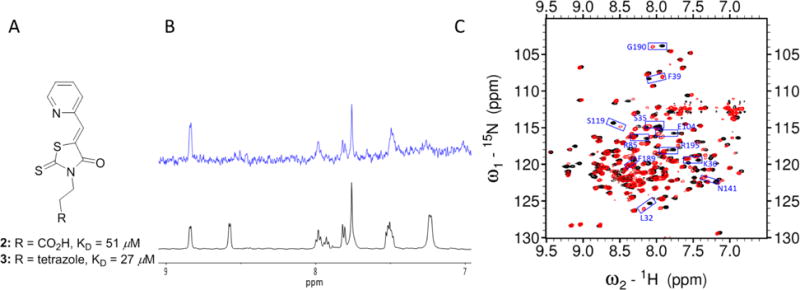
Characterization of initial compounds 2–3. A) Chemical structures and KD values of compounds 2–3 in the fluorescence quenching assays. B) STD-NMR experiment results: 1H spectra (black) and the STD spectrum (blue) of compound 3. Note that the 1H NMR of compound 3 indicated a mixture of two rotamers with estimation of 1:1 ratio. One of the two rotamers binds favorably to apo pa-HemO. C) 1H,15N-HSQC NMR results of compound 3. Black: apo pa-HemO; Red: compound 3 mixed with apo pa-HemO.
