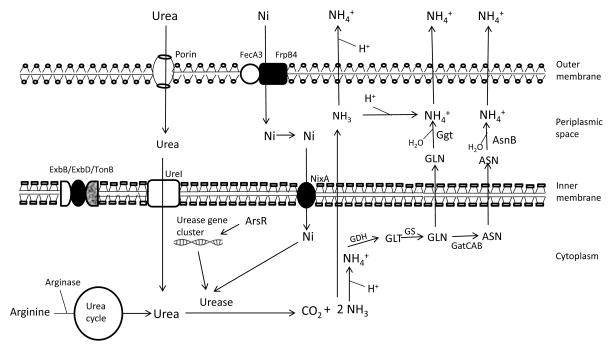
Figure 2.
The metallo-enzyme, urease requires nickel (Ni) for its activity. Ni slowly enters the outer membrane through porin (outer membrane protein) and actively enters through FecA3/FrpB4 proteins anchored in outer membrane and crosses inner membrane through NixA (inner membrane protein). However, in acidic pH more Ni is required for increased activity of urease and for the active transport of essential molecules (Ni), ExbD provides energy to the outer membrane. Urea enters the outer membrane through the porin and inner membrane through UreI (found in inner membrane) which opens in acid exposure of bacteria. Endogenously produced by arginase in urea cycle (during in vitro survival) and exogenously entered urea molecules are hydrolyzed by urease to ammonia (NH3) and carbon dioxide (CO2). ArsR regulates urease gene cluster expression. Ammonia (NH3) produced is diffused out and binds with proton (H+) leading to the neutralization of acidic pH. However, NH3 is also converted to ammonium (NH4+) inside the cell (cytoplasm and periplasm) which is toxic to the bacteria. The cytoplasmic NH4+ is assimilated to glutamate (GLT) by GDH and to glutamine (GLN) by GS. GLN can diffuse to periplasmic space or can be converted to aspergine (ASN) by GatCAB aminoacyl-tRNA amidotransferase (GatCAB) and then can diffuse to periplasmic space. GLN and ASN bind with water molecule and led to the formation of NH4+ and catalyzed by Ggt and AsnB respectively. Finally the NH4+ exits to the bacterial cell and increases the pH that leads to the survival of bacteria at acidic pH.