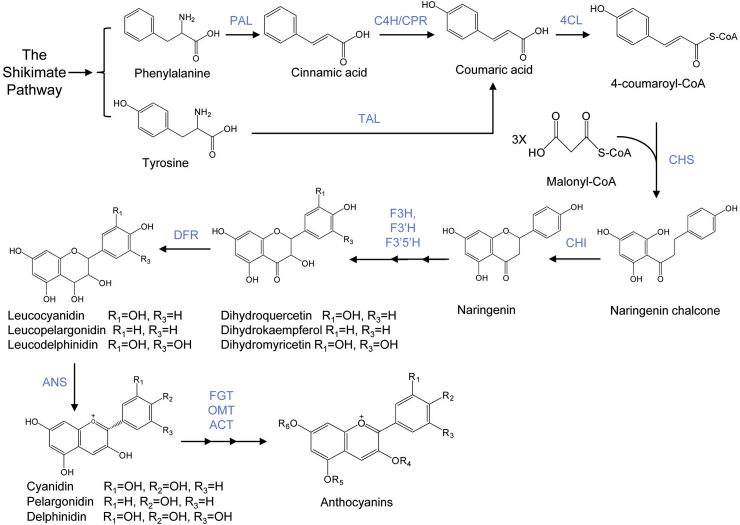
Fig. 2.
The pathway of anthocyanin biosynthesis in plants. The general precursor phenylalanine or tyrosine derived from the shikimate pathway is converted to 4-coumaroyl-CoA through the phenylpropanoid pathway or the combined effect of TAL and 4CL, respectively. One molecule of 4-coumaroyl-CoA is condensed with three molecules of malonyl-CoA to form one molecule of naringenin chalcone, which is subsequently converted to naringenin by CHI. Naringenin, the major intermediate compound, undergoes various hydroxylation to form diverse anthocyanidins. Further glycosylation and other decorations act on the anthocyanidin compounds to generate anthocyanins. Abbreviations: PAL, phenylalanine ammonia lyase; C4H, cinnamate 4-hydroxylase; CPR, cytochrome P450 reductase; TAL, tyrosine ammonia lyase; 4CL, 4-coumaroyl-CoA ligase; CHS, chalcone synthase; CHI, chalcone isomerase; F3H, flavanone 3-hydroxylase; F3′H, flavonoid 3′-hydroxylase; F3′5′H, flavonoid 3′, 5′-hydroxylase; DFR, dihydroflavonol reductase; ANS, anthocyanidin synthase; FGT, flavonoid glucosyltransferase; OMT, O-methyltransferase; ACT, anthocyanin acyltransferase.