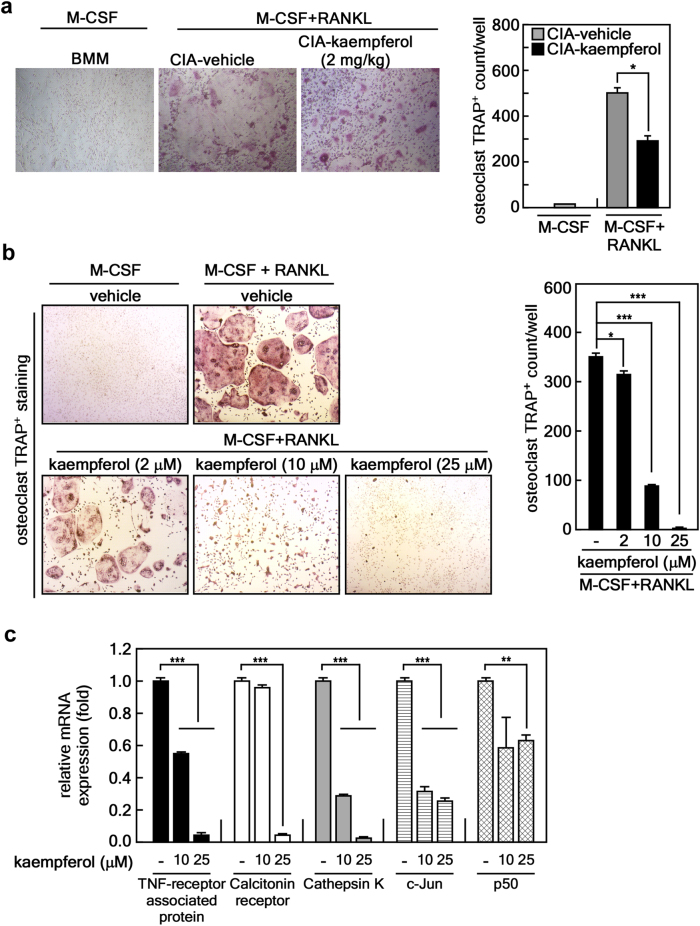
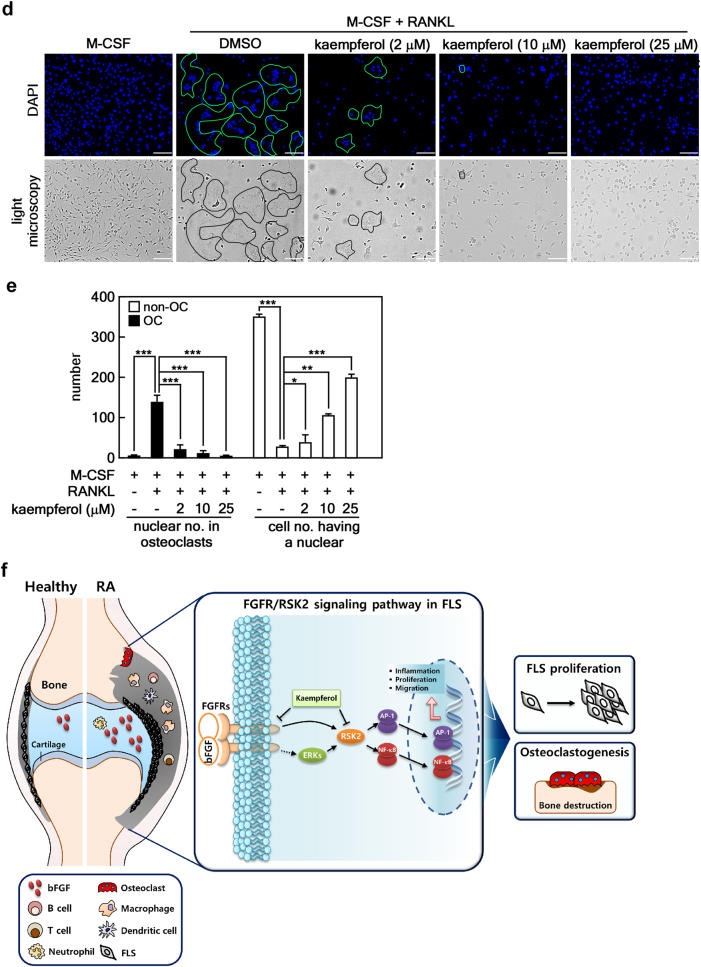
Fig. 6. Kaempferol inhibits osteoclast differentiation.
a Inhibitory effects of kaempferol on ex vivo osteoclast formation. The BMMs obtained from CIA + vehicle and CIA + kaempferol mice were analyzed in terms of the osteoclast formation induced by M-CSF or M-CSF + RANKL. TRAP+ osteoclasts (≥3 nuclei/TRAP+ cell) were counted. Photographs (×100) are representative of TRAP staining obtained from each mouse group (CIA + vehicle, n = 3; CIA + kaempferol, n = 3), and values obtained from the whole well of a 48-well plate are presented as means ± SEM. *p < 0.05. b Inhibitory effects of kaempferol on in vitro osteoclast formation. Naïve murine BMMs were subjected to osteoclast differentiation by combinational stimulation of kaempferol, M-CSF, and RANKL as indicated. TRAP+ osteoclasts ( ≥ 3 nuclei/TRAP+ cell) were counted. Photographs (×100) are representative of TRAP staining obtained from three independent experiments, and values obtained from the whole well of a 48-well plate are represented as means ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001. c Inhibitory effects of kaempferol on osteoclast-specific genes. Naïve murine BMMs stimulated with M-CSF/RANKL and indicated doses of kaempferol for 4 days, and mRNA levels of indicated osteoclast-specific genes were measured by real-time PCR. Data were obtained from three independent experiments, and values are represented as means ± SEM. **p < 0.01; ***p < 0.001. d Representative photographs of morphological osteoclast analysis. The indicated area shows a multinucleated giant osteoclast cell body after treatment with kaempferol and M-CSF/RANL, as indicated. Scale bars, 40 μm. e Inhibitory effects of kaempferol on osteoclast differentiation. The total nuclear number of multinucleated (≥3 nuclei) giant cells with the phenotypic features of osteoclasts and the number of cells with a single nucleus were counted. Data were obtained from three independent experiments using a four-chamber slide, and values are represented as means ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001. f Schematic of the signaling pathway targeted by kaempferol for the inhibition of osteoclast differentiation. bFGF-FGFR3 interaction transduces activation signaling to RSK2, resulting in hyperplasia by the induction of inflammation, FLS proliferation, and cell migration through NF-κB and AP-1. Eventually, the macrophages in synovium differentiate to bone absorbing osteoclasts. Thus, the dual targeting of kaempferol on both FGFR3 and RSK2 may prevent RA in humans