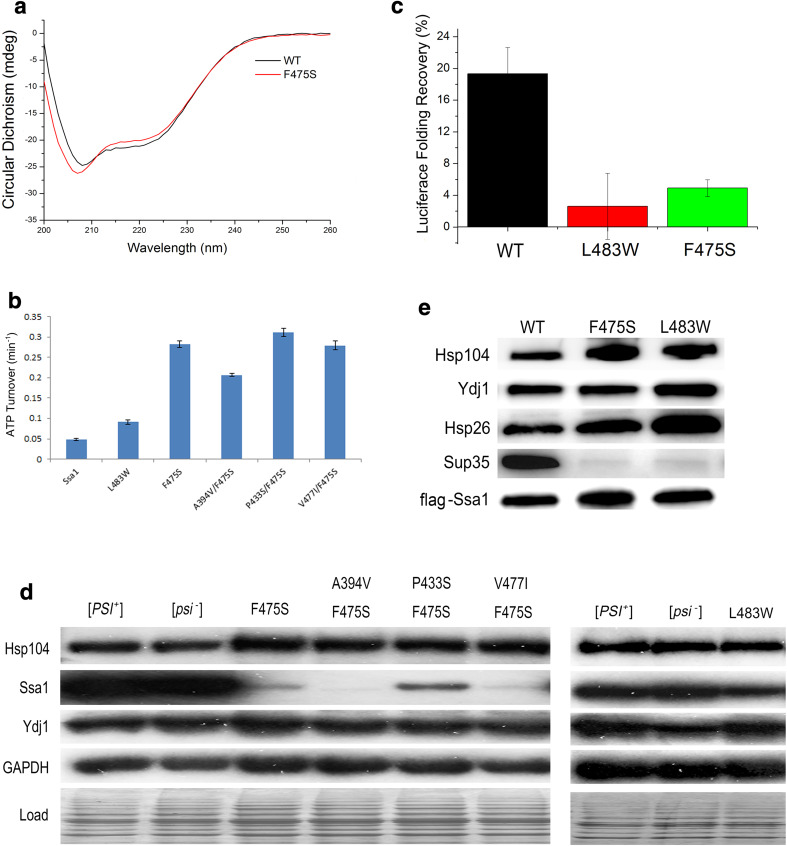
Fig. 4.
Disruption of SBD alters functions of Ssa1. a Secondary structure monitored by far-UV CD spectra for full-length Ssa1 at 30 °C. b ATPase activity of the full-length Ssa1 proteins. The unit of the ATP turnover rate is min−1. The values shown are the mean of four replicates from independent measurements and the error bars represent the standard deviation. c Luciferase refolding activity of F475S and L483W mutation yeast strains. Fresh cultures were shifted to 37 °C for 30 min before 45 °C denaturation for 1 h. Denatured luciferase cultures were recovered at 25 °C for a 1 h period. Cycloheximide was added to prevent protein synthesis during the recovery period. d Chaperone abundance of the Hsp70 machinery. Western blotting was performed to assess the expression levels of Hsp104, Ssa1, and Ydj1. GAPDH and a stained SDS-PAGE ran under the same conditions were used as loading controls. e F475S and L483W substitutions alter the Ssa1 interactions with clients. FLAG-tagged Ssa1 was pulled down from G402 cells and probed for Hsp104, Ydj1, Hsp26, and Sup35. FLAG-Ssa1 was used as loading control