Abstract
Introduction
Solid pseudopapillary Carcinoma (SPC) is a rare pancreatic Tumor with variable, usually low, malignancy potential. Howewer, several SPC are associated with aggressive behavior, local vascular infiltration, organ invasion, distant metastasis, and can be unresectable. Irreversible Electroporation (IRE) is an emerging non-thermal ablation technique for the treatment of locally advanced pancreatic carcinoma. We report the results of four year disease-free follow-up in a case of locally advanced unresectable SPC treated with IRE.
Presentation of case
A 24-year female patient with SPC of the pancreas underwent IRE during laparotomy under general anesthesia with intubation. Computed Tomography (CT) showed complete tumor thrombosis of splenic vein, encasement of celiac artery and mesenteric vein. Six insertions of 3–4 electrodes per insertion were performed. One month-CT-control showed shrinkage of the tumor. 6 months-post-treatment imaging showed complete regression of the mass, patent Splenic/mesenteric veins, absence of local recurrence or distant metastasis. Post treatment CTs at 12-18-24-30-36-42-48 months follow-up confirmed absence of local or distant recurrence.
Discussion
Surgery is the first choice curative treatment of SPC. Howewer aggressive surgery (duodeno-pancreasectomy) in unresectable cases, may have a high risk of recurrences, morbidities and death, and bring concerns about endocrine and exocrine insufficiency in a young patient. In these cases, IRE could be a safe and effective alternative treatment and could realize, in selected cases, the condition for a radical surgery, and a bridge to R-0 resection.
Conclusions
IRE could represent an effective alternative therapy to surgery in local advanced, unresectable SPC.
Keywords: Pancreatic neoplasm, Solid papillary carcinoma, Intraoperative ultrasound, Irreversible electroporation, Case report
Highlights
-
•
Solid pseudopapillary Carcinoma (SPC) is a rare pancreatic Tumor with possible local vascular infiltration, distant metastasis, and can be unresectable.
-
•
Irreversible Electroporation (IRE) is a non-thermal ablation technique for locally advanced pancreatic neoplasms.
-
•
There is no case of SPC treated with IRE reported in the literature.
-
•
IRE could be a safe and effective alternative treatment and could be a bridge to R-0 resection.
1. Introduction
Solid pseudopapillary Neoplasm (SPN) is a rare pancreatic tumor with variable, usually low, malignancy potential, that represents only 1–2% of pancreatic tumors [1]. Most SPN are discovered incidentally after cross section images exams such as Ultrasound (US), computed tomography (CT) or magnetic resonance (MR) [2]. Usually, SPN is an indolent disease with a good prognosis. Over 90% of patients with a solitary pancreatic lesion are cured by complete excision [3]. Howewer, SPN is not always indolent. Several forms are associated with aggressive behavior [4,5]. Several authors indicate two different types of SPN: the benign type called Solid Papillary Tumor (SPT) and the malignant one defined as Solid Papillary Carcinoma (SPC) of the pancreas [6].
Irreversible electroporation (IRE) is an emerging non-thermal ablation technique that allows tissue ablation without the potential detrimental heat-effects on tissue surrounding the tumor. It delivers short electrical pulses through probes inserted into the tissue in order to modify the cell membrane permeability that results in cells' death [7]. IRE showed high efficacy in the treatment of locally advanced pancreatic carcinoma and also in other neoplasms of the pancreas [8,9]. One of the major advantages of this technique is that it can be used in tumors that are in close proximity to peri-pancreatic structures without risk of vascular trauma or biliary damage [7].
We report the case of a young patient affected from an aggressive large SPC of the pancreas with locally invasive behavior, successfully treated with IRE, with a long term disease-free-survival follow-up.
The work has been reported in line with the SCARE criteria [10].
2. Case report
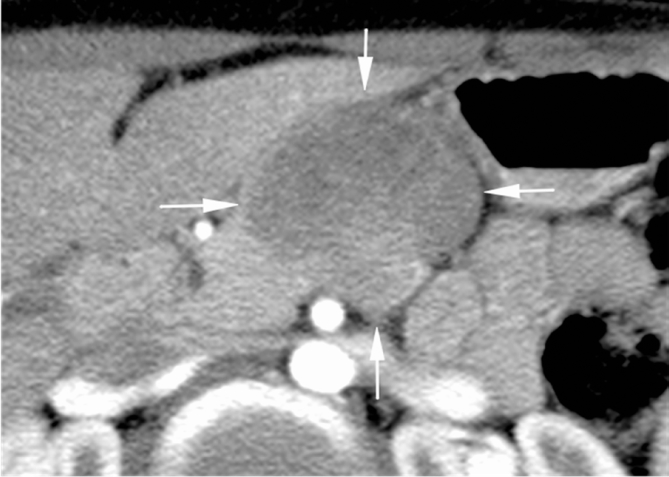
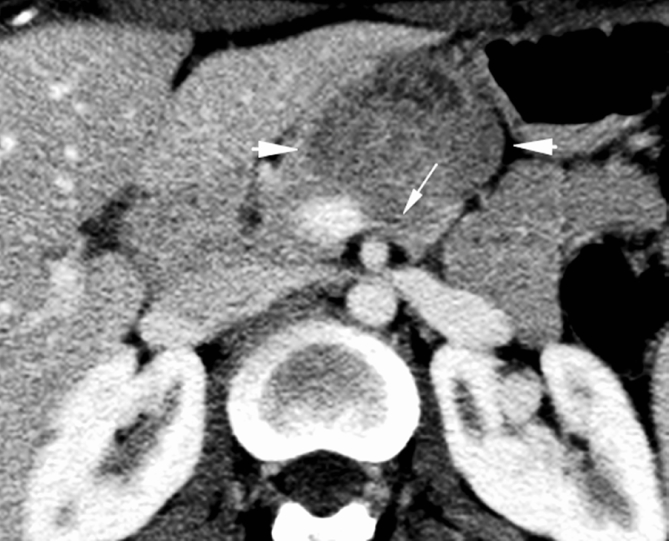
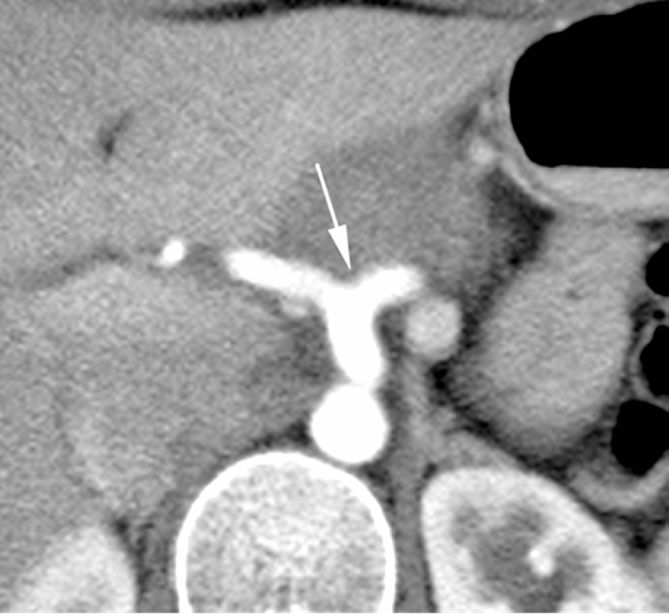
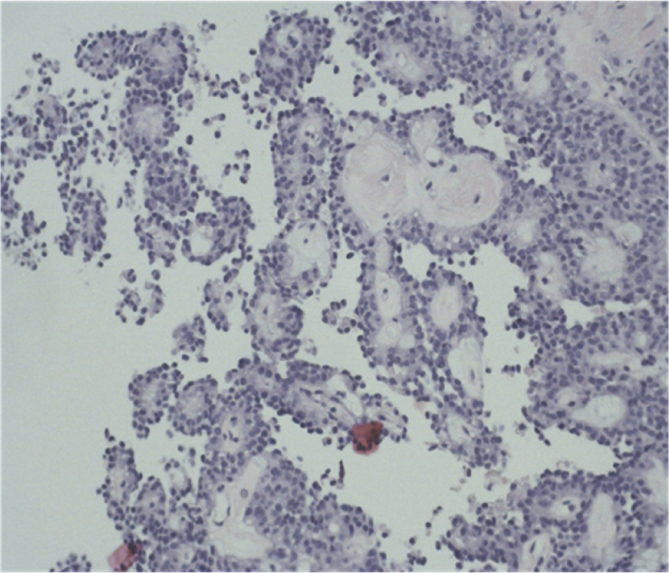
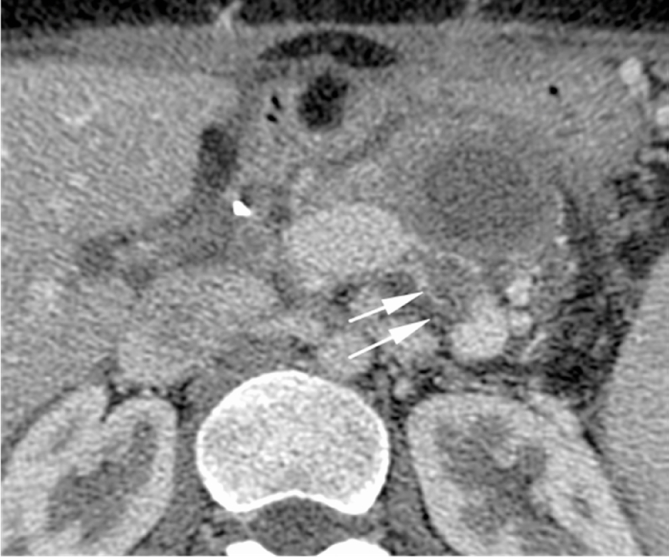
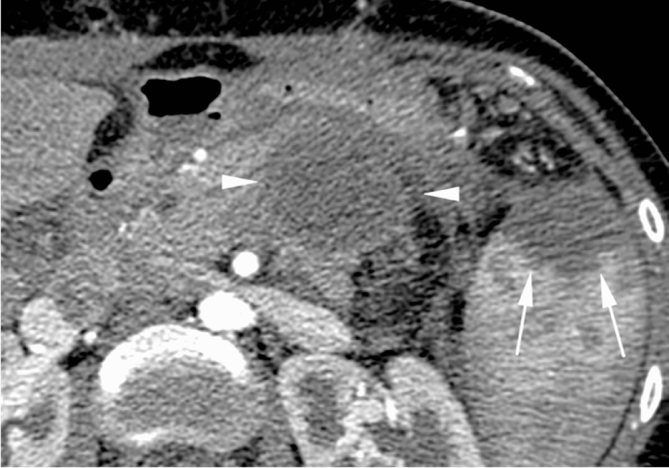
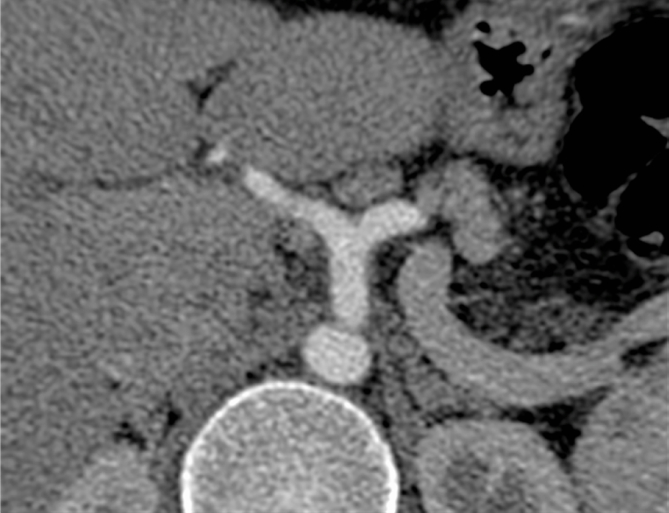
24 years young Female was admitted at our Division of Surgery – A. Tortora Cancer Hospital, in the late august 2013, because of a solid upper abdominal mass detected at a US examination performed the day before. In the last 2 months she complained epigastric pain, nausea, lack of appetite and weight loss. At admission she showed a tender abdomen, absence of peritonism, no clear palpable mass, a slight tenderness at deep palpation of the epigastrium. She showed normal breathing, normal blood pressure (120/70 mmHg) and heart rate (80/min), normal diuresis and feces without sign of rectorraggia. Blood tests showed slight anemia (Hgb = 10.8%) and increased amylase level (256 U/L). All the other routine blood tests were within normal ranges. There was mild increase of Carbohydrate Antigen 19-9; (65 U/ml) while all the other tumor markers were normal. Abdominal US, total body enhanced CT and MR showed a solid mass (diameters = 65 × 55 × 50 mm) involving the pancreatic body and isthmus (Fig. 1), complete tumor thrombosis of the middle and distal portions of splenic vein (Fig. 2), encasement of celiac artery (Fig. 3), and infiltration of the mesenteric vein wall. Imaging did not show any distant metastasis to lymphnodes, liver, lung or other sites. The patient underwent Percutaneous US guided biopsy that showed pancreatic “Solid Papillary Adenocarcinoma”(Fig. 4).
Fig. 1.
CT scan shows a large solid mass of pancreatic body and tail (arrows) that displaces nearby vascular structures.
Fig. 2.
CT scan shows complete tumor thrombosis of splenic vein (arrows).
Fig. 3.
CT scan shows encasement of celiac artery (arrow).
Fig. 4.
US guided biopsy shows: pancreatic “Solid Papillary Adenocarcinoma”.
The oncology-Surgical team judged the tumor unresectable or possibly resectable with vascular resection and reconstruction. The partial resection was also considered an acceptable aim, so that the patient was advised for surgery. Howewer, after informed consent, with discussion of the high risks of surgical intervention, consequences of major resection and possible incomplete excision of the tumor, the patient refused surgical therapy. As alternative and less invasive treatment, ablation of the tumor by IRE was offered. The patient accepted this second choice and informed consent to undergo IRE treatment was obtained.
PROCEDURE: The patient underwent laparotomy under general anesthesia with intubation. In order to avoid strong muscle contractions induced by electric pulses, the myorelaxant cisatracurium besylate (Nimbex®, GlaxoSmithKline, Brentford, United Kingdoms) was used. After laparotomy the gastro-colic omentum was opened to reach and expose the pancreatic tumor. In order to schedule the next steps, an accurate intraoperative Color doppler US examination was performed with a commercially available equipment (EPIC 7 - Philips Healthcare-United States), high frequency (7–15 MHz) intraoperative probe (L15-7io – Philips Healthcare-United States), to assess the tumor size and shape, and its relationship with large vessels, gastrointestinal tract, liver and spleen. Then, we started the IRE procedure. Multiple electrodes (up to 4 per insertion) connected independently to the electric pulses generator (NanoKnife® - Angiodynamics, Inc., NY, United States) were inserted inside the tumor at a distance of 2–2.5 cm apart. The first insertion was realized in the deeper part of the mass consistent with the splenic vein thrombus. After electrodes deposition, the electric pulses, automatically synchronized to each patient's cardiac cycle, were delivered. After completed the pulses delivery in that part of the tumor, the electrodes were partially pulled back or completely pull out, and reinserted in other portions of the tumor. This procedure was repeated five times in order to cover all the other parts of the tumor. In total, six insertions of 3–4 electrodes were performed. The whole procedure lasted 160 minutes. At the end of IRE procedure, an intraoperative post-treatment biopsy of the mass was performed. The abdomen of the patient was closed. No intraoperative complication was registered. Histology on post-IRE biopsy showed a preserved architecture of the tumor with diffuse apoptosis and signs of “spotty necrosis”. The patient had an uneventful recovery and left the Hospital 5 days after treatment.
FOLLOW-UP: Follow-up included CT examination after 1 month as short term control of efficacy. Thereafter, clinical examination, routine laboratory tests, abdominal US every three months and CT every 6 months were scheduled. During the follow-up the patients was asymptomatic and never complained of any pain or undesired effect.
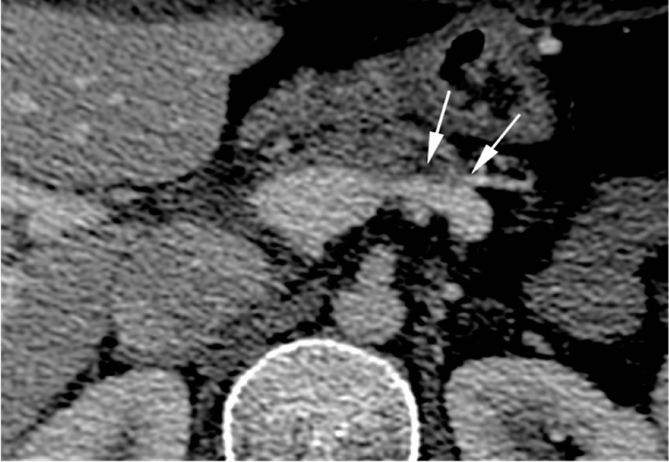
One month post-treatment CT showed: “decreased size of the mass, (Fig. 5), patent splenic vein with partial avascular “bland” thrombosis of the left portion (Fig. 5), patent mesenteric and portal vein, an organized hematoma next to the site of ablation and small splenic infarction” (Fig. 6). Three months post-treatment evaluation by PET/MR showed absence of pathological uptake and apparently absence of residual tumor or recurrence. 6 months-post-treatment CT showed complete regression of the mass (Fig. 7), normal aspect of celiac artery (Fig. 7), patent splenic/mesenteric veins (Fig. 8). Post treatment CTs at – 12–18 - 24–30 - 36–42 - 48 months follow-up confirmed the same findings and did not show any local or distant recurrence. At the time we wrote this paper, the patient is completely asymptomatic and apparently disease-free at 48 months follow-up.
Fig. 5.
One-month post-treatment CT shows “shrinkage of the tumor, partial bland thrombosis of the splenic vein (arrows), patent mesenteric and portal vein.
Fig. 6.
One-month CT shows an organized hematoma (arrowheads) next to the site of ablation and subcapsular splenic infarction (arrows).
Fig. 7.
Enhanced CT at 6 months after treatment shows complete regression of the mass and normal celiac artery.
Fig. 8.
Enhanced CT at 6 months after treatment shows patent splenic and portal vein (arrows). The splenic vein shows slight distortion of the left portion. Left portion of pancreatic body appears irregular but with normal Wirsung duct and absence of lesions or pathologic lymphnodes.
3. Discussion
SPC occurs mostly in women (87.8%), between 20 and 40 years with a mean age of 28 years, and the female-to-male ratio is 9:1 [1]. Over 90% of patients with a solitary pancreatic lesion are cured by complete surgical excision. Howewer, local recurrence or distant metastases can occur in a significant number of patients [even after R-0 resection [5]. Several forms of SPC shows aggressive behavior and can be the cause of death of a young patient [[3], [4], [5],11]. Local vascular and organ invasion or distant metastasis are found in 9–15% at first diagnosis. Criteria which distinguish the solid pseudopapillary carcinoma, by the 2010 WHO classification, are angioinvasion, perineural invasion and deep invasion of the surrounding pancreatic parenchyma [12].
Surgery is the first choice curative treatment and is considered indicated even if a R1 resection can be achieved. In advanced cases (SPC) aggressive surgery (duodeno-pancreasectomy) is mandatory since an incomplete resection may have a high risk of recurrence and death [4,5,11]. Howewer, as most patients are young with a long life expectancy, and shall be cured after resection, concerns about endocrine and exocrine insufficiency are important, because they can impair quality of life. Minor concerns could be splenectomy in a young patient, high surgical risks in case of high demolitive intervention with vascular reconstruction, large incisions and poor aesthetic outcome. Under this context, minimally invasive parenchyma-preserving procedures or systemic therapy would be advisable.
Systemic Chemotherapy with cisplatin and 5-fluorouracil (5-FU) or gemcitabine in treatment of SPC remains controversial, and except for several anedoctal studies no substantial benefit has been reported [13]. Radiotherapy has shown no demonstrable response [13].
RadioFrequency Ablation (RFA) is a minimally invasive loco-regional technique that have been used in the treatment of locally advanced pancreatic carcinoma (LAPC) [14]. Howewer, the heat generated from RFA could damage the numerous critical structures next to pancreas (duodenum, main biliary ducts, portal vein, hepatic artery). In the treatment of LAPC, RFA demonstrated a very high morbidity and mortality rates up to 40% and 25% respectively and only minimal benefit on survival of the patients [15,16]. In our opinion, thermal ablation, either RFA or Microwave ablation, should not be considered in locally advanced stage SPC.
Irreversible electroporation (IRE) is a new, non-thermal ablation technique for the treatment of parenchymal organ tumors. The first preclinical studies were published in 2007 [7,17]. Rubinsky et al. in their experimental study from 2007, described three main features which give IRE an advantage over other ablative techniques:
-
1.
the remarkable effectiveness of ablation;
-
2.
IRE ablation does not suffer from the heat sink effect and thus the cells in the vicinity of the vessels undergo ablation equally with the rest of the ablated part of the tissue;
-
3.
IRE ablation retains functionality of the blood vessels, bile ducts, urinary tract and nerves which are located in the ablation area [17].
The United States Food and Drug Administration have approved the technique for use in the pancreas. In a large series of patients with LAPC by Martin et al. [18] IRE improved overall survival, progression-free survival, and distant progression-free survival when comparing patients who underwent IRE with those who underwent chemotherapy and/or radiation therapy alone. In most of the papers dealing with IRE of LAPC in literature, the authors show an high success rate of the procedure to control local progression of the tumor [19,20].
Based on these previous experiences with IRE in pancreatic cancer, we decided to offer IRE treatment to our patient with a locally advanced SPC. Actually, the young age and the reliable favourable prognosis due to the histological type of tumor indicated a minimally invasive approach by IRE as possible effective treatment. Our aim was the complete ablation of the tumor. In this view, we adopted the strategy to treat in open surgery with multiple applications of electrodes in order to be sufficiently confident to have achieved the complete ablation. High resolution intraoperative US played a substantial role to guide electrodes' repositioning into the tumor. In our opinion, the limitations of Percutaneous approach (low resolution US probes, interposition of gastrointestinal tract, unsufficient definition of vessels) would have decrease the opportunity of complete ablation and could have increased the risk of complications. The complete resolution of the space occupying lesions and a normal appearance of the pancreas and Wirsung's duct at 6-months follow-up CT after treatment, confirms the high efficacy of IRE as local ablation technique. The patient is experiencing a very good quality of life without any discomfort from the previous intervention. 48 months follow-up disease-free-survival is certainly a good result; howewer, late and very late recurrences of SPC have been reported, so that a longer follow-up will be needed to assess the radicality of IRE treatment.
4. Conclusions
In conclusion, in cases with poor indications for surgical resection, IRE could be a safe and highly effective alternative therapy for advanced stage pancreatic SPC, and could be a bridge treatment to radical surgery.
Ethical approval
Our institution does not require institutional review board or other approval for single case report which are deidentified. Consent Written informed consent was obtained from the patient for this study. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Institutional review board approval or ethics committee approval was not required as this was a retrospective de-identified case report.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contribution
Conception or design of work: LT.
Acquisition, analysis, or interpretation of data for the work: LT, GB, RF, SB, CA.
Drafting the work or revising it critically for important intellectual content: LT, AN.
Final approval of the version to be published: LT.
Conflict of interest
The authors declare no conflict of interest and have no acknowledgements.
Guarantor
LT MD.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Acknowledgement
None.
References
- 1.Law J.K., Ahmed A., Singh V.K., Akshintala V.S., Olson M.T., Raman S.P. A systematic review of solid-pseudopapillary neoplasms: are these rare lesions? Pancreas. 2014;43:331–337. doi: 10.1097/MPA.0000000000000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baek J.H., Lee J.M., Kim S.H. Small (<or=3 cm) solid pseudopapillary tumors of the pancreas at multiphasic multidetector CT. Radiology. 2010;257:97–106. doi: 10.1148/radiol.10092089. [DOI] [PubMed] [Google Scholar]
- 3.Huang H.L., Shih S.C., Chang W.H., Wang T.E., Chen M.J., Chan Y.J. Solid-pseudopapillary tumor of the pancreas: clinical experience and literature review. World J. Gastroenterol. 2005;11:1403–1409. doi: 10.3748/wjg.v11.i9.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tipton S.G., Smyrk T.C., Sarr M.G. Malignant potential of solid pseudopapillary neoplasm of the pancreas. Br. J. Surg. 2006;93:733–737. doi: 10.1002/bjs.5334. [DOI] [PubMed] [Google Scholar]
- 5.Sperti C., Berselli M., Pasquali C., Pastorelli D., Pedrazzoli S. Aggressive behaviour of solid-pseudopapillary tumor of the pancreas in adults: a case report and review of the literature. World J. Gastroenterol. 2008;14:960–965. doi: 10.3748/wjg.14.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Q Yin, Wang M., Wang C., Wu Z., Yuan F., Chen K., Tang Y., Zhao X., Miao F. Differentiation between benign and malignant solid pseudopapillary tumor of the pancreas by MDCT. Eur. J. Radiol. 2012 Nov;81(11):3010–3018. doi: 10.1016/j.ejrad.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Al-Sakere B., André F., Bernat C., Connault E., Opolon P., Davalos R.V., Rubinsky B., Mir L.M. Tumor ablation with irreversible electroporation. PLoS One. 2007;2:2–11. doi: 10.1371/journal.pone.0001135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moir J., White S.A., French J.J., Littler P. Manas DM2Systematic review of irreversible electroporation in the treatment of advanced pancreatic cancer. Eur. J. Surg. Oncol. 2014;40:1598–1604. doi: 10.1016/j.ejso.2014.08.480. [DOI] [PubMed] [Google Scholar]
- 9.Orcutt S., Kis B., Malafa M. Case report: irreversible electroporation for locally advanced pancreatic cancer. Int. J. Surg. Case Rep. 2017;1(40):54–57. doi: 10.1016/j.ijscr.2017.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S., Orgill D.P., for the SCARE Group The SCARE Statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;27:187–189. doi: 10.1016/j.ijsu.2016.01.094. [DOI] [PubMed] [Google Scholar]
- 11.Nishihara K., Nagoshi M., Tsuneyoshi M., Yamaguchi K., Hayashi I. Papillary cystic tumors of the pancreas. Assessment of their malignant potential. Cancer. 1993;71:82–92. doi: 10.1002/1097-0142(19930101)71:1<82::aid-cncr2820710114>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 12.Lüttges J. What's new? The 2010 WHO classification for tumours of the pancreas. Pathologe. 2011;32:332–336. doi: 10.1007/s00292-011-1515-2. [DOI] [PubMed] [Google Scholar]
- 13.Matsunou H., Konishi F. Papillary-cystic neoplasm of the pancreas. A clinicopathologic study concerning the tumor aging and malignancy of nine cases. Cancer. 1990;65:283–291. doi: 10.1002/1097-0142(19900115)65:2<283::aid-cncr2820650217>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 14.Spiliotis J.D., Datsis A.C., Michalopoulos N.V. Radiofrequency ablation combined with palliative surgery may prolong survival of patients with advanced cancer of the pancreas. Langenbecks Arch. Surg. 2007;392:55–60. doi: 10.1007/s00423-006-0098-5. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y., Tang Z., Fang H. High operative risk of cool-tip radiofrequency ablation for unresectable pancreatic head cancer. J. Surg. Oncol. 2006;94:392–395. doi: 10.1002/jso.20580. [DOI] [PubMed] [Google Scholar]
- 16.Figueroa-Barojas P., Bakhru M.R., Habib N.A. Safety and efficacy of radiofrequency ablation in the management of unresectable bile duct and pancreatic cancer: a novel palliation technique. J. Oncol. 2013;2013 doi: 10.1155/2013/910897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edd J.F., Horowitz L., Davalos R.V., Mir L.M., Rubinsky B. In vivo results of a new focal tissue ablation technique: irreversible electroporation. IEEE Trans. Biomed. Eng. 2006;53:1409–1415. doi: 10.1109/TBME.2006.873745. [DOI] [PubMed] [Google Scholar]
- 18.Martin R.C., Kwon D., Chalikonda S., Sellers M., Kotz E., Scoggins C. Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: safety and efficacy. Ann. Surg. 2015;262:486–494. doi: 10.1097/SLA.0000000000001441. [DOI] [PubMed] [Google Scholar]
- 19.Young S.J. Irreversible electroporation and the pancreas: what we know and where we are going? World J. Gastrointest. Surg. 2016;7:138–144. doi: 10.4240/wjgs.v7.i8.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rombouts S.J., Vogel J.A., van Santvoort H.C., van Lienden K.P., van H.R., Busch O.R. Systematic review of innovative ablative therapies for the treatment of locally advanced pancreatic cancer. Br. J. Surg. 2015;102:182–193. doi: 10.1002/bjs.9716. [DOI] [PubMed] [Google Scholar]