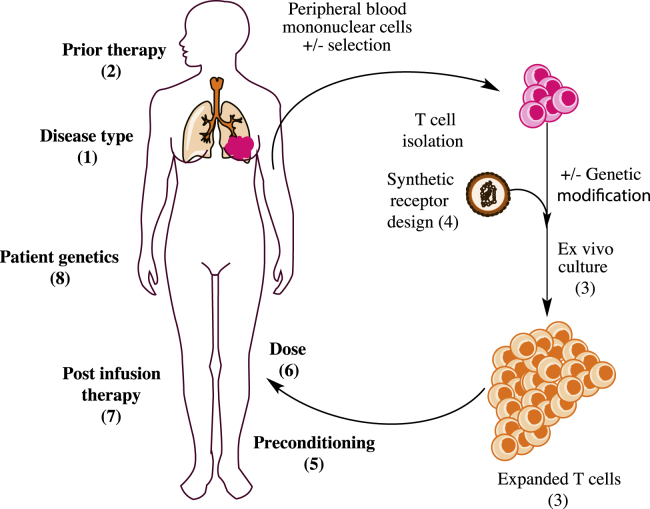
Figure 2.
Variables Impacting Efficacy of T Cell Therapy
Many recipient and product factors are thought to impact the efficacy of adoptive T cell therapy. Although effects have largely not been tested in human trials, in vitro, preclinical in vivo, and human trial evidence implicate parameters at all points of the T cell therapy “life cycle”: (1) disease type, (2) prior therapy, (3) expansion culture conditions (e.g., cytokine and length of culture), including T cell phenotype of the product, (4) synthetic antigen receptor design, (5) pre-conditioning regimen and tumor burden, (6) T cell dose, and (7) post-infusion therapy. (8) Recipient genetics, especially those related to immunity, likely also impact efficacy.