Abstract
Purpose
To demonstrate clearing of chronic corneal opacities and improvement of visual acuity with the use of BostonSight prosthetic replacement of the ocular surface ecosystem (PROSE) treatment in ocular surface disease.
Observations
We undertook retrospective analysis of the medical records of a series of patients who underwent PROSE treatment from August 2006 to December 2014. Patients were referred for ocular surface disease of various etiologies. Primary inclusion criterion was corneal opacity that improved with PROSE treatment. Patients were excluded if topical steroids or adjuvant therapy used once PROSE treatment was initiated. Underlying disease, prior treatment, clinical presentation, and clinical course were extracted from the medical record. Four patients are included in this series. There were three females and one male; median age at time of treatment initiation was 30 years (range = 0.5–58 years). Median duration of PROSE treatment at time of retrospective analysis was 3.5 years (range = 1–8 years). Two cases had corneal opacification in the context of neurotrophic keratopathy: a unilateral case due to presumed herpes simplex keratitis and a bilateral case due to congenital corneal anesthesia associated with familial dysautonomia. One case had corneal opacity from exposure related to seventh nerve palsy, and one had corneal opacification associated with recurrent surface breakdown, neurotrophic keratopathy, and limbal stem deficiency of uncertain etiology. After consistent wear of prosthetic devices used in PROSE treatment for support of the ocular surface, visual acuity improved and clearing of the opacities was observed, without use of topical steroids or adjuvant therapy.
Conclusions and importance
These cases demonstrate clearing of chronic corneal opacity with PROSE treatment for ocular surface disease. This clearing can occur with no adjuvant therapy, suggesting that restoration of ocular surface function and integrity allows for corneal remodeling.
Keywords: PROSE treatment, Ocular surface disease, Opacity, Corneal scar, Dry eye syndrome, Scleral lenses, Scleral prosthetic devices
1. Introduction
Corneal transparency is heavily dependent upon the highly complex and regular spatial order of the collagen fibrils within the stromal layer.1, 2, 3, 4, 5 Transparency can become compromised when the cornea is exposed to infection, trauma, chronic inflammation or ulceration.6,7 When any of these occur, a series of complex wound healing mechanisms ensue, in order to protect the cornea and its integrity.1,8, 9, 10, 11 After injury or insult, the stroma begins to remodel and becomes significantly different in structure and composition from that of the normal corneal stroma. One way is different, is that it lacks matrix order. This lack of matrix order in the remodeling stroma may contribute to opacity formation.1,11
Even when lack of matrix order in the remodeling stroma is believed to contribute to corneal opacity after injury or insult, animal studies have shown that over time (months to years), collagen fibril size become progressively more regular and the stromal fibrils also become more organized in arrangement,12 which are believed to be contributing processes in the potential return of corneal transparency.2,12
Surgical intervention such as phototherapeutic keratectomy (PTK), lamellar keratoplasty, and penetrating keratoplasty (PK) are typically undertaken for chronic opacities that limit vision. Typically these are classified as “scars.” Surgical intervention has inherent risk of infection, and in the case of penetrating keratoplasty, bleeding and rejection. There are substantial resource requirements involved in post-operative care, including office visits and medications. While surgery may lead to anatomic success, contact lens rehabilitation or spectacle wear may nevertheless be required, with a time course for visual rehabilitation being as long as one year.13
PROSE treatment (developed by BostonSight, Needham, MA, www.bostonsight.org), uses FDA-approved custom designed prosthetic devices to support or replace impaired ocular surface system functions that protect and enable vision.14
While visual rehabilitation of corneal opacity with RGP contact lenses,13 scleral lenses14, 15, 16, 17, 18, 19 and PROSE treatment20, 21, 22, 23 has been described, we believe that a role for therapeutic lenses for the clearing or resolution of chronic corneal opacity in the setting of ocular surface disease is not appreciated and has never been reported. We present a series of four cases of resolution of chronic corneal opacity and improvement of visual acuity with PROSE treatment.
2. Methods
This study was a retrospective interventional case series. This retrospective medical record review of patients with dry eye syndrome was deemed exempt from IRB review by New England Institutional Review Board, as under 10–125, for research involving the collection or study of existing data or records if the information is recorded by the investigator in such a manner that subjects cannot be identified.
We undertook retrospective analysis of the medical records of a series of patients who underwent PROSE treatment from August 2006 to December 2014. Patients were referred for dry eye syndrome of various etiologies. Primary inclusion criterion was corneal opacity that improved with PROSE treatment. Patients were excluded if topical steroids or any other topical and or surgical approach was used once PROSE treatment was initiated. Underlying disease, prior treatment, clinical presentation, and clinical course were extracted from the medical record.
PROSE treatment involves the design and custom fabrication of FDA-approved prosthetic devices for therapeutic use on a daily wear basis, made out of two high gas-permeable fluorosilicone-acrylate polymers (Dk 85 or 127 × 10−11·cm2 ·ml O2/s ml mm Hg [ISO/Fatt]). All devices were designed and fabricated using a proprietary CAD/CAM technology to customize the bearing surface of the device haptic to align with the supporting sclera and a transitional and optic portion designed to vault the cornea. The device is filled with artificial tears at the time of application and removed for cleaning and disinfection. Assessment of physiological function with prosthetic devices used in PROSE treatment included evaluation of corneal clearance and haptic alignment, fluid ventilation, corneal status, and subjective tolerance after 1, 3–4, and 6–8 hours of prosthetic device wear. Routine photo-documentation of corneal findings using an RS-1000 Zoom Slit Lamp digital photo unit with a mounted Nikon D200 camera was an integral part of clinical assessment. Patients returned for evaluation of medical status and monitoring of device function at 1, 3 and 6 months after devices were dispensed and yearly after that.
3. Case report #1
An 11-year-old male was referred to BostonSight in November 2006 with an 18-month history of persistent epithelial defect (PED) in the left eye. Past ocular history was also significant for strabismic amblyopia in the left eye.
There was incidental report of tree branch injury to the left eye. He was referred with a presumptive diagnosis of herpes simplex neurotrophic keratitis. Previous treatments included two failed amniotic membrane grafts. His medications at time of referral included 400 mg oral acyclovir twice daily, autologous serum tears four times daily and sodium chloride hypertonic ointment nightly.
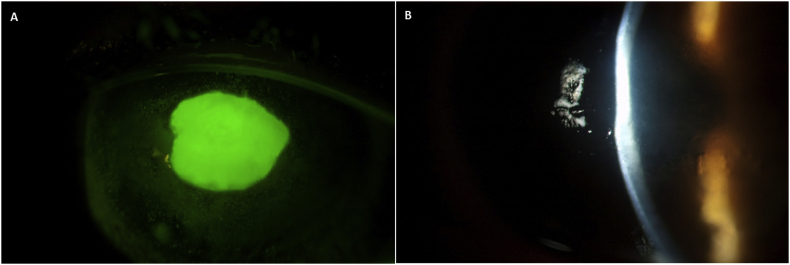
Entering uncorrected visual acuity at initial consultation was 20/20+ in the right eye and 20/400 in the left eye, with improvement to 20/70 with pinhole (PH). On slit lamp examination, a central epithelial defect measuring 3 mm × 3.5 mm was noted (Fig. 1A), with 2 + central haze and 20–30% stromal thinning (Fig. 1B). Corneal sensitivity using a Luneau Cochet-Bonnet Aesthesiometer was measured at 0.5 cm in the right eye and 7 mm in the left eye. The remainder of the eye examination was unremarkable.
Fig. 1.
Acute persistent epithelial defect (PED) and stromal thinning as observed in patient described in case report #1. A) PED with B) 20–30% stromal thinning in the left eye in 2006.
He was treated with a custom-fabricated device for the left eye that was worn overnight with prophylactic use of preserved-free moxifloxacin antibiotic and daily removal and disinfection as previously described.18 The PED healed two weeks after initiating PROSE treatment. Best-corrected visual acuity once the PED healed improved to 20/70−2 PH 20/50−2.
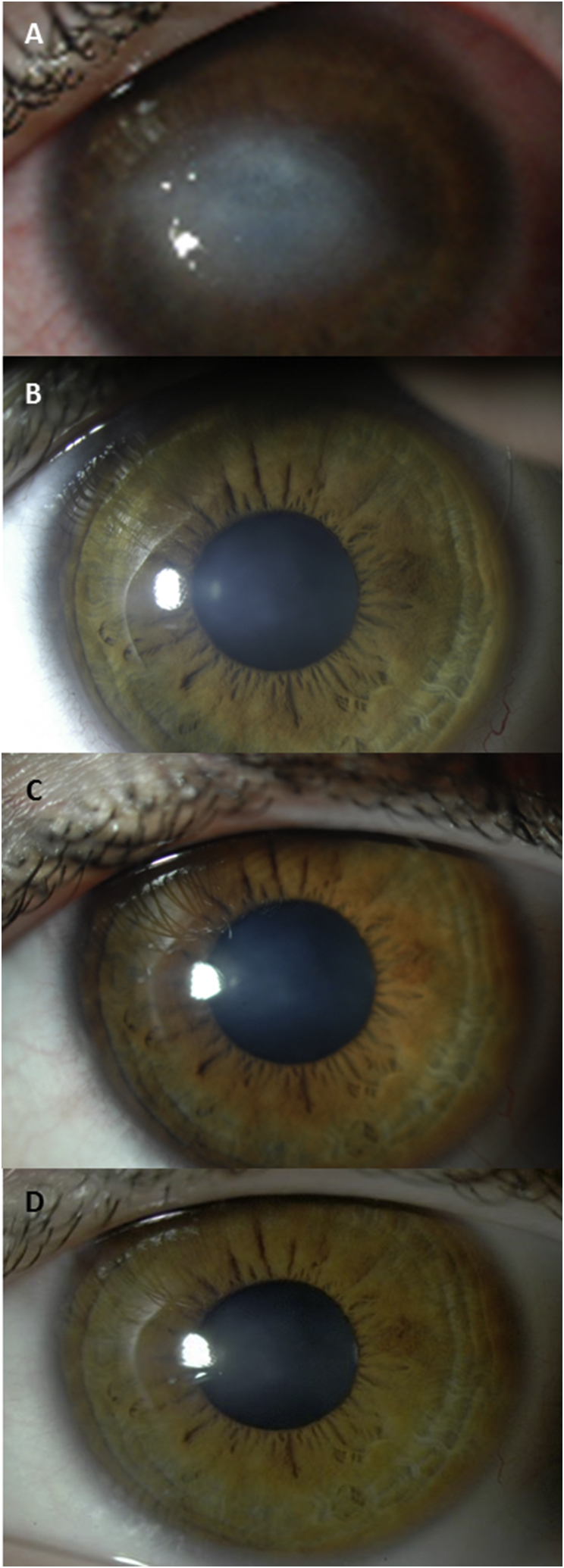
This patient was monitored over the next six years of daily wear of devices used in BostonSight PROSE treatment. No additional topical agents, such as corticosteroid or anti-viral agents were used, nor was any surgical intervention undertaken. There was no recurrence of surface breakdown or episode of epithelial of stromal keratitis. There was clearing of corneal opacity observed over the subsequent eight years of PROSE treatment (Fig. 2). Best corrected visual acuity in the left eye (with history of strabismic amblyopia) remained at 20/70 PH 20/50.
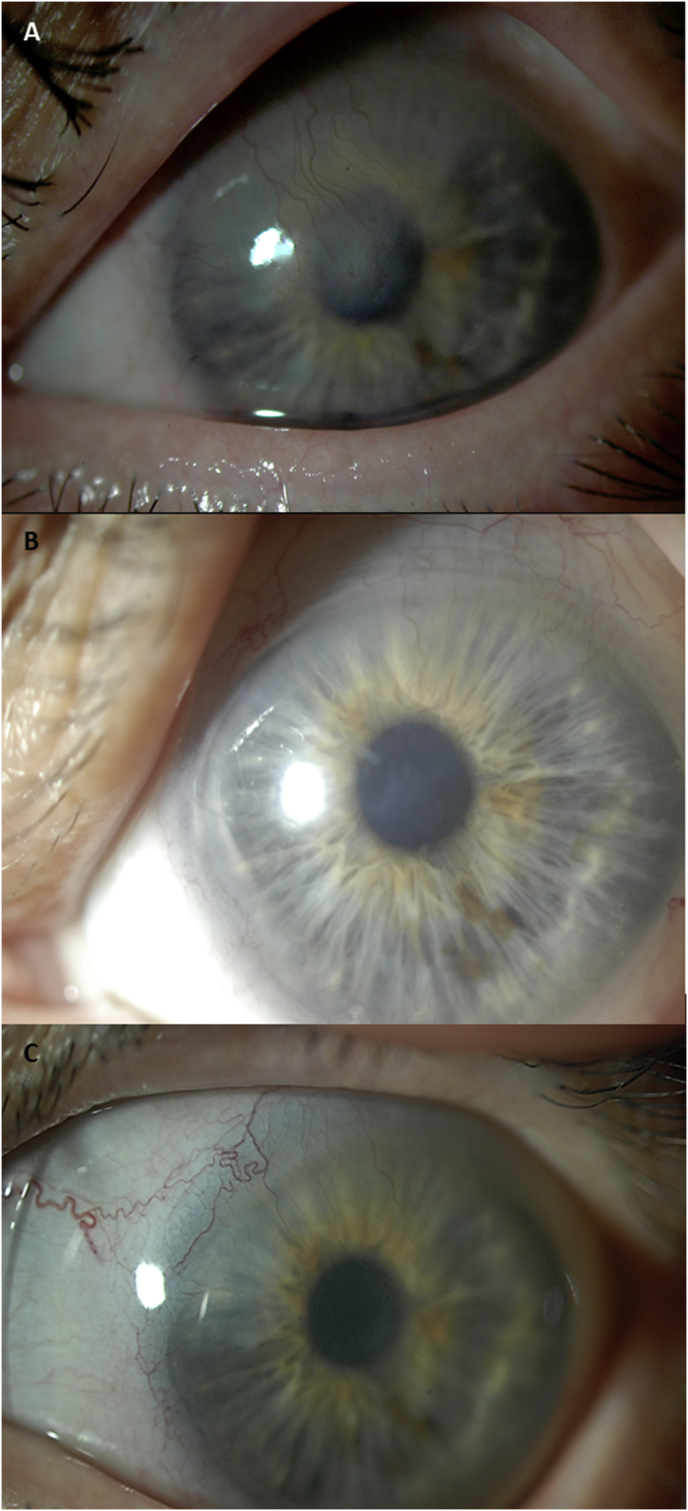
Fig. 2.
Corneal opacity regression in a case of presumed Herpes Simplex Virus Type 1 (HSV1). A) Dense corneal opacity after persistent epithelial defect in 2007 with best corrected visual acuity (BCVA) 20/200 at baseline, and after prosthetic replacement of the ocular surface ecosystem (PROSE) treatment B) in 2010 with BCVA 20/70, C) in 2012 and, D) 2013 with BCVA 20/50 as observed in patient described in case report #1.
4. Case report #2
A 6-month old female was referred to BostonSight in September 2008. A diagnosis of congenital corneal anesthesia from Hereditary Sensory and Autonomic Neuropathy Type III, Familial Dysautonomia (once called Riley-Day syndrome) was made at three months of age. She had a history of superficial keratitis in both eyes and ulceration and persistent epithelial defects (PED) in the right eye that healed after three amniotic membrane grafts. At the time of the initial consultation visual function at the fixate and follow level could be confirmed for the left eye only.
Penlight and ophthalmoscope with blue light and Wratten #12 yellow filter examination showed corneal staining in the right eye more than left eye with no epithelial defects present at the time of consultation. There was corneal opacification with thinning in the right eye (Fig. 3A). A retinoscopic red reflex was obtained for the left eye but was undetectable in the right eye. PROSE treatment was undertaken for both eyes with power determination based on cycloplegic retinoscopy of the left eye.
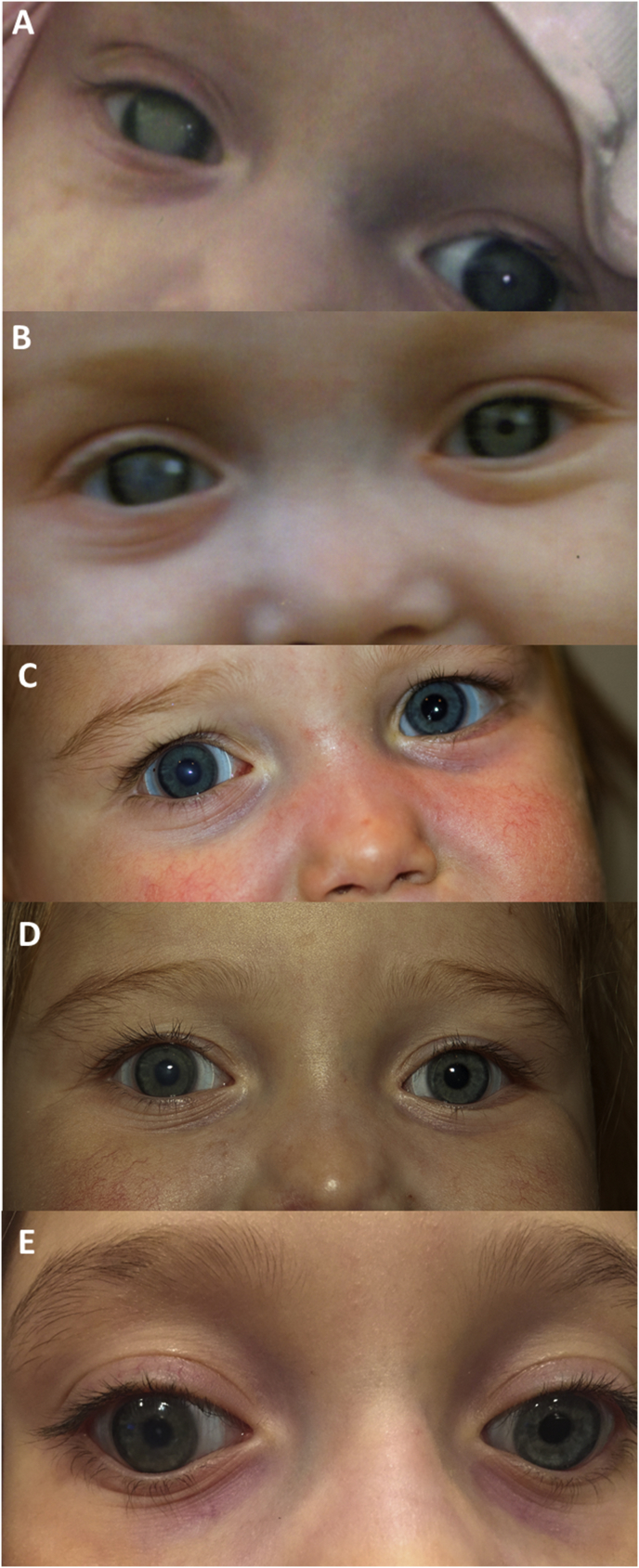
Fig. 3.
Corneal opacity regression in a case of Familial Dysautonomia. A) Dense corneal opacity after persistent epithelial defect in 2008 at baseline, B) after 3 months of prosthetic replacement of the ocular surface ecosystem (PROSE) treatment, C) in 2010, D) in 2011 and, E) 2014 as observed in patient described in case report #2.
At the visit three months later, her mother described improved visualization of right pupil (Fig. 2B). Fix and follow vision could be confirmed for left eye only.
She returned seven months later and a retinoscopic reflex could be obtained (Fig. 3B). Topical atropine had been prescribed back in September 2008 by her referring doctors for the right eye to aid in image formation around the opacity and discontinued eleven months after. Patching occlusion of the left eye for amblyopia treatment required upper extremity restraint and was abandoned. Eventually an occlusive soft contact lens was used over the device in her left eye for occlusion therapy.19
After 18 months of PROSE treatment, a decrease in the corneal opacity in the right eye was observed (Fig. 3C). Full cycloplegic refractive error, determined by retinoscopy, was prescribed for each prosthetic device. She could fixate, follow, and reach out for an object using each eye alone. Continued clearing of opacity and stability of the ocular surface was noted over the subsequent six years (Fig. 3D and E).
5. Case report #3
A 49-year-old female was referred to BostonSight in June 2013 with history of chronic exposure and superficial keratitis of the right eye associated with facial nerve palsy after head trauma at age 15. Previous treatments of the right eye included: partial tarsorrhaphy, upper lid weight, superior and inferior punctal occlusion, and nightly sodium chloride hypertonic ointment.
Entering corrected distance visual acuity was 20/50 + PH 20/30 in the right eye, 20/30 PH 20/15 in the left eye. Slit lamp evaluation revealed a partial tarsorrhaphy with incomplete blink, upper lid weight, and corneal haze with associated neovascularization involving the visual axis (Fig. 4A).
Fig. 4.
Corneal opacity regression in a case of chronic exposure. A) Opacification and neovascularization as a result of chronic exposure in at baseline, and B) January 2014, after prosthetic replacement of the ocular surface ecosystem (PROSE) treatment, as observed in patient described in case report #3.
PROSE treatment was initiated with improvement of visual acuity to 20/20 in the right eye. At one year, a decrease of opacification with regression of neovascularization was observed (Fig. 4B). Best-corrected visual acuity in the right eye was 20/20+.
After two years of daily wear of a PROSE device for support of the ocular surface, visual acuity remained 20/20 in the right eye with continued regression of opacity and neovascularization.
6. Case report #4
A 58-year-old female was referred to BostonSight in 2013 for bilateral corneal opacification, worse in the left eye, due to recurrent surface breakdown, neurotrophic keratopathy, and limbal stem cell deficiency of unknown etiology. There was a 20-year history of daily soft contact lens wear. Corneal erosion with ulceration first occurred eleven months prior to consultation. Prior treatment included: bandage soft contact lenses, amniotic membrane graft, autologous serum tears, loteprednol 0.5%, preservative free artificial tears, gel drops, and topical moxifloxacin.
Entering distance visual acuities with a cosmetic soft contact lens for visual rehabilitation was 20/50 PH 20/30 in the right eye. Unaided visual acuity in the left eye was 20/400, PH 20/100. Examination of the right eye was significant for mild, diffuse injection, superior corneal pannus, and trace nuclear sclerotic cataract. Examination of the left eye was significant for lower lid punctal occlusion, opacity and neovascularization of the superior cornea approaching the visual axis (Fig. 5A), inferior corneal pannus, and trace nuclear sclerotic cataract.
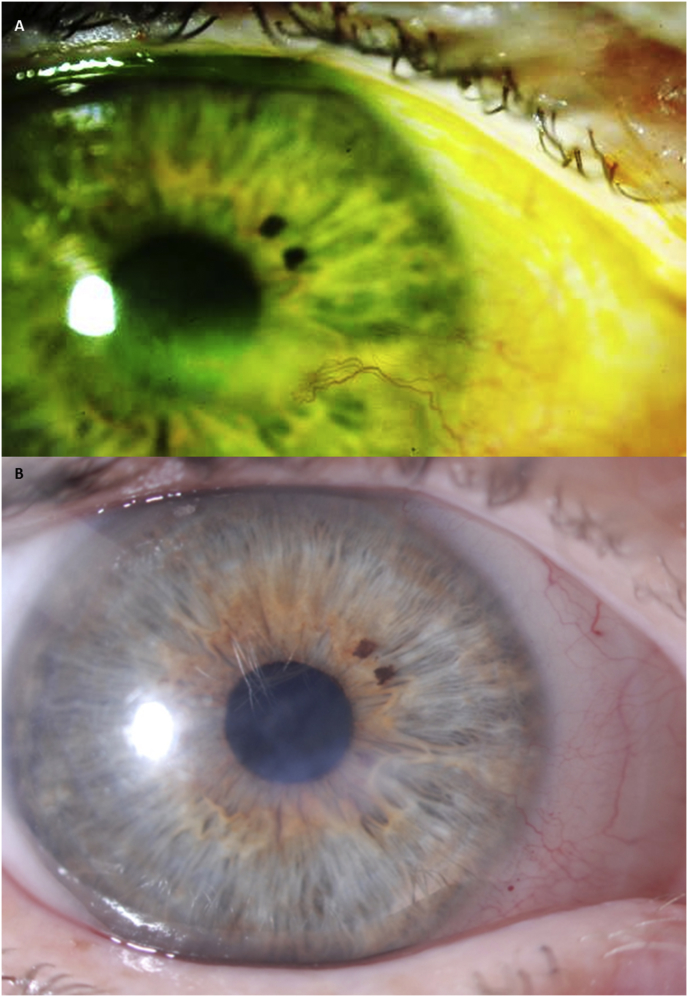
Fig. 5.
Corneal opacity regression in a case of neurotrophic keratopathy and limbal stem cell deficiency of unknown etiology. A) Opacification and neovascularization in the left eye, at baseline November 2013, and B) January 2014, and C) May 2014 after prosthetic replacement of the ocular surface ecosystem (PROSE) treatment as observed in patient described in case report #4.
PROSE treatment was undertaken with improvement of distance visual acuity to 20/30+2 in the right eye and 20/40−2 PH 20/30 in the left eye.
After 3 months, entering distance visual acuity with PROSE devices was 20/25+2 in the right eye and 20/40+2 PH 20/25−2 in the left eye. Slit lamp evaluation of the left eye revealed ghosting of the corneal neovascularization and reduction in central opacity (Fig. 5B). Improvements in neovascularization and opacity continued at 7 months of daily wear of a PROSE device (Fig. 5C).
After one year of treatment, distance visual acuity improved to 20/20−1 in the right eye and 20/30−2 PH 20/25−3 in the left eye.
7. Discussion
Globally, corneal opacity accounts for 1.5–2.0 million new cases of monocular blindness every year.24 Treatment for corneal opacity includes topical corticosteroids, contact lenses, and surgical approaches to improve transparency. Soft contact lenses, and to a larger degree, rigid gas-permeable contact lenses also mask irregular astigmatism from corneal scarring by providing a smooth refracting surface, thereby improving visual acuity. However, the extent of visual improvement is usually limited by the corneal opacification.13,25,26 If the cornea returns to its original transparency, the potential for visual improvement would increase considerably.
Prompt treatment and choice of treatment modalities after corneal trauma, corneal defects, infections, or chronic exposure, are key to maintaining corneal integrity and limiting corneal opacity. Among the many treatment options, autologous serum (AS) has been found to provide an environment to promote healing as it contains essential tear components in comparable concentrations to natural tears, i.e., vitamin A, epidermal growth factor, fibronectin, and transforming growth factor-β, which are important substances for corneal and conjunctival integrity.27, 28, 29, 30 Initial opacity formation can be minimized because of the reduction of inflammation and because of stromal remodeling supported by serum factors and nerve growth factor that are able to promote proliferation and differentiation of limbal corneal epithelium cells.31 Additionally, amniotic membrane (AM) grafting modulates wound healing by promoting epithelialization while suppressing stromal inflammation, angiogenesis and scarring.32 Amniotic membrane stromal matrix exerts a direct anti-scarring effect on ocular tissue fibroblasts by suppressing TGF-β signaling.33 While the benefits of AS and AM grafting on wound healing have been documented in the literature, there is little evidence that these agents contribute to clearing of chronic corneal opacity.
We propose that regression in corneal opacity occurs because PROSE treatment provides an environment that supports healing and maintains integrity of the ocular surface. Once ocular surface integrity is established and inflammation reduced, stromal remodeling can occur through a prolonged process of synthesis, degradation and resynthesis.1,8, 9, 10, 11 Clearing of corneal opacity has been of feature of cases depicted in other reports of PROSE treatment from this center,18,21,34 but clearing of opacity was not the specific focus of those reports. In those depicted cases, PROSE treatment was used in conjunction with other interventions, such as topical corticosteroids, that might account for clearing of opacity and improvement in vision.
We believe this series warrants attention precisely because these four cases cleared with PROSE treatment alone. In each case, the opacity was chronic and not related to an acute insult, such as microbial keratitis, and there was no use of topical steroid or other intervention to reduce inflammation and promote clearing.
Cases 1 and 2 presented here show the most striking improvement in opacity. It may be that the pediatric cornea has greater potential for remodeling of corneal opacity. The implications for the treatment of children and for understanding corneal wound healing and remodeling warrant further investigation.
A limitation of this case series is the lack of cohort design for selection of cases. Prospective interventional case series of defined cohorts have shown that PROSE treatment results in improvement in visual acuity and visual function at six months17,35 and that there is maintenance of improved visual function at 5 years.23 A prospective study of the course of opacity in a cohort of all patients undergoing PROSE treatment would allow for identification of predictors and correlates of clearing, such as age of patient, underlying disease, and the use of adjuvants such as topical corticosteroids or autologous serum tears.
It is recognized that PROSE treatment is costly. An economic appraisal of treatment at found a cost-effectiveness ratio that is well below the cut-off values for other health care technologies.36 It is also worthy of note that PROSE treatment has been found to be clinically effective compared to penetrating keratoplasty, which is an alternative option for corneal opacity or scarring,37 although that study was confined to cases of keratoconus.
In summary, the clearing of chronic corneal opacity and improvement in visual acuity with PROSE treatment, without use of any adjuvant therapy, in this small series of patients with ocular surface disease, suggests that restoration of ocular surface function and integrity allows for corneal remodeling. This finding is worthy of attention by clinicians and scientists alike.
Patient consent to publication
In each case, written informed consent regarding risks and benefits of BostonSight PROSE treatment was obtained from the patient or legal guardian. Consent was also obtained from all patients to share all details related to the case reports presented herein. Accordingly, all guidelines were followed to ensure HIPAA compliance, and we adhered to the Declaration of Helsinki and applicable federal and state laws.
Funding
No funding or grant support.
Conflict of interest
The following authors have no financial disclosure: A.C., C.R., D.J., K.C.
K. G. Carrasquillo, C. Remington, and D. S. Jacobs are a salaried employees of BostonSight, Needham, MA, where BostonSight® PROSE treatment was developed. A. Cressey is a former salaried employee of BostonSight. None of the authors have a proprietary interest in PROSE treatment or the prosthetic devices used in PROSE treatment.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgments
None.
References
- 1.Hassell J.R., Birk D.E. The molecular basis of corneal transparency. Exp Eye Res. 2010;91(3):326–335. doi: 10.1016/j.exer.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen S., Mienaltowski M.J., Birk D.E. Regulation of corneal stroma extracellular matrix assembly. Exp Eye Res. 2015;133:69–80. doi: 10.1016/j.exer.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fullwod N.J. Collagen fibril orientation and corneal curvature. Structure. 2004;12:169–170. doi: 10.1016/j.str.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 4.Meek K.M., Boote C. The use of x-ray scattering techniques to quantify the orientation and distribution of collagen in the corneal stroma. Prog Retin Eye Res. 2009;28:369–392. doi: 10.1016/j.preteyeres.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Kim A., Zhou C., Lakshman N., Petroll W.M. Corneal stromal cells use both high- and low-contractility migration mechanisms in 3-D collagen matrices. Exp Cell Res. 2012;318:741–752. doi: 10.1016/j.yexcr.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boote C., Dennis S., Huang Y.F., Quantock A.J., Meek K.M. Lamellar orientation in human cornea in relation to mechanical properties. J Struct Biol. 2005;149:1–6. doi: 10.1016/j.jsb.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Anderson K., El-Sheikh A., Newson T. Application of structural analysis to the mechanical behavior of the cornea. J R Soc Interface. 2004;1:3–15. doi: 10.1098/rsif.2004.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steele C. Corneal wound healing: a review. Optom Today. 1999;40:28–32. [Google Scholar]
- 9.Agrawal V.B., Tsai R.F. Corneal epithelial wound healing. Indian J Ophthalmol. 2003;51:5–15. [PubMed] [Google Scholar]
- 10.Eraslan M., Toker E. Mechanisms of corneal wound healing and its modulation following refractive surgery. Mamara Med J. 2009;22:169–178. [Google Scholar]
- 11.Cintron C., Hassinger L.C., Kublin C.L., Cannon D.J. Biochemical and ultrastructural changes in collagen during corneal wound healing. J Ultra Res. 1978;65:13–22. doi: 10.1016/s0022-5320(78)90017-5. [DOI] [PubMed] [Google Scholar]
- 12.Cintron C., Covington H.I., Kublin C.L. Morphologic analyses of proteoglycans in rabbit corneal scars. Invest Ophthalmol Vis Sci. 1990;31:1789–1798. [PubMed] [Google Scholar]
- 13.Singh K., Jain D., Kunal T. Rehabilitation of vision disabling corneal opacities: is there hope without corneal transplant? Contact Lens Anterior Eye. 2013;36:74–79. doi: 10.1016/j.clae.2012.10.085. [DOI] [PubMed] [Google Scholar]
- 14.Romero-Rangel T., Stavrou P., Cotter J.M. Gas permeable scleral lens therapy in ocular surface disease. Am J Ophthalmol. 2000;130:130–141. doi: 10.1016/s0002-9394(00)00378-0. [DOI] [PubMed] [Google Scholar]
- 15.Romero-Jimenez M., Flores-Rodriquez P. Utility of a semi-scleral contact lens design in the management of the irregular corena. Contact Lens Anterior Eye. 2013;36(3):146–150. doi: 10.1016/j.clae.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Rosenthal P., Croteau A. Fluid-ventilated, gas-permeable scleral contact lens is an effective option for managing severe ocular surface disease and many corneal disorders that would otherwise require penetrating keratoplasty. Eye Contact Lens. 2005;31:130–134. doi: 10.1097/01.icl.0000152492.98553.8d. [DOI] [PubMed] [Google Scholar]
- 17.Stason W.B., Razavi M., Jacobs D.S. Clinical benefits of the Boston ocular surface prosthesis. Am J Ophthalmol. 2010;149(1):54–61. doi: 10.1016/j.ajo.2009.07.037. [DOI] [PubMed] [Google Scholar]
- 18.Lim P., Ridges R., Jacobs D.S., Rosenthal P. Treatment of persistent corneal epithelial defect with overnight wear of prosthetic device for the corneal surface. Am J Ophthalmol. 2013;156(6):1095–1101. doi: 10.1016/j.ajo.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Michaud L., Carrasquillo K.G. Piggy-back cosmetic contact lens as an occlusion therapy in a patient with familial dysautonomia. Eye Contact Lens. 2010;36(6):367–370. doi: 10.1097/ICL.0b013e3181f57aed. [DOI] [PubMed] [Google Scholar]
- 20.Lee J.C., Chiu G.B., Bach D. Functional and visual improvement with prosthetic replacement of the ocular surface ecosystem scleral lenses for irregular corneas. Cornea. 2013;32(12):1540–1543. doi: 10.1097/ICO.0b013e3182a73802. [DOI] [PubMed] [Google Scholar]
- 21.Papakostas T.D., Le H.G., Chodosh J., Jacobs D.S. Prosthetic replacement of the ocular surface ecosystem as treatment for ocular surface disease in patients with a history of Stevens-Johnson syndrome/toxic epidermal necrolysis. Ophthalmology. 2015;122(2):248–253. doi: 10.1016/j.ophtha.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 22.DeLoss K.S., Le H.G., Gire A. PROSE treatment for ocular chronic graft-versus-host disease as a clinical network expands. Eye Contact Lens. 2016;42(4):262–266. doi: 10.1097/ICL.0000000000000186. [DOI] [PubMed] [Google Scholar]
- 23.Agranat J.S., Kitos N.R., Jacobs D.S. Prosthetic replacement of the ocular surface ecosystem: impact at 5 years. Br J Ophthalmol. 2016;100(9):1171–1175. doi: 10.1136/bjophthalmol-2015-307483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burton M.J. Corneal blindness: prevention, treatment and rehabilitation. Community Eye Health J. 2009;22(71):33–35. [PMC free article] [PubMed] [Google Scholar]
- 25.McClintic S.M., Srinivasan M., Mascarenhas J. Improvement in corneal scarring following bacterial keratitis. Eye (Lond) 2013;27(3):443–446. doi: 10.1038/eye.2012.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Titiyal J.S., Das A., Dada V.K., Tandon R., Ray M., Vajpayee R.B. Visual performance of rigid gas permeable contact lenses in patients with corneal opacity. CLAO J. 2001;27(3):163–165. [PubMed] [Google Scholar]
- 27.Ogawa Y., Okamoto S., Mori T. Autologous serum eye drops for the treatment of severe dry eye in patients with chronic graft-versus-host disease. Bone Marrow Transplant. 2003;31:579–583. doi: 10.1038/sj.bmt.1703862. [DOI] [PubMed] [Google Scholar]
- 28.Young A.L., Cheng A.C., Ng H.K., Cheng L.L., Leung G.Y., Lam D.S. The use of autologous serum tears in persistent corneal epithelial defects. Eye. 2004;18:609–614. doi: 10.1038/sj.eye.6700721. [DOI] [PubMed] [Google Scholar]
- 29.Geerling G., MacLennan S., Hartwig D. Autologous serum eye drops for ocular surface disorders. Br J Ophthalmol. 2004;88(11):1467–1474. doi: 10.1136/bjo.2004.044347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee Y.K., Lin Y.C., Tsai S.H., Chen W.L., Chen Y.M. Therapeutic outcomes of combined topical autologous serum eye drops with silicone-hydrogel contact lenses in the treatment of corneal persistent epithelial defects: a preliminary study. Contact Lens Anterior Eye. 2016;39(6):425–430. doi: 10.1016/j.clae.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Semeraro F, Forbice E, Braga O, et al., Evaluation of the efficacy of 50% autologous serum eye drops in different ocular surface pathologies. BioMed Res Int. http://dx.doi.org/10.1155/2014/826970. [DOI] [PMC free article] [PubMed]
- 32.Liu J., Sheha H., Fu Y., Liang L., Tseng S.C. Update on amniotic membrane transplantation. Expet Rev Ophthalmol. 2010;5(5):645–661. doi: 10.1586/eop.10.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tseng S.G., Li D., Ma X. Suppression of transforming growth factor isoforms, TGF-β receptor II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J Cell Physiol. 1999;179:325–335. doi: 10.1002/(SICI)1097-4652(199906)179:3<325::AID-JCP10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 34.Cressey A.M., Carasquillo K.G., Jacobs D.S. Management of vascularized limbal keratitis (VLK) with prosthetic replacement of the ocular surface system. Eye Contact Lens. 2012;38(2):137–140. doi: 10.1097/ICL.0b013e31823bafbc. [DOI] [PubMed] [Google Scholar]
- 35.Baran I., Bradley J.A., Alipour F., Rosenthal P., Le H.G., Jacobs D.S. PROSE treatment of corneal ectasia. Contact Lens Anterior Eye. 2012;35(5):222–227. doi: 10.1016/j.clae.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Shepard D.S., Razavi M., Stason W.B. Economic appraisal of the Boston ocular surface prosthesis. Am J Ophthalmol. 2009;148(6):860–868. doi: 10.1016/j.ajo.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 37.DeLoss K.S., Fatteh N.H., Hood C.T. Prosthetic replacement of the ocular surface ecosystem (PROSE) scleral device compared to keratoplasty of the treatment of corneal ectasia. Am J Ophthalmol. 2014;158(5):974–982. doi: 10.1016/j.ajo.2014.07.016. e2. [DOI] [PubMed] [Google Scholar]