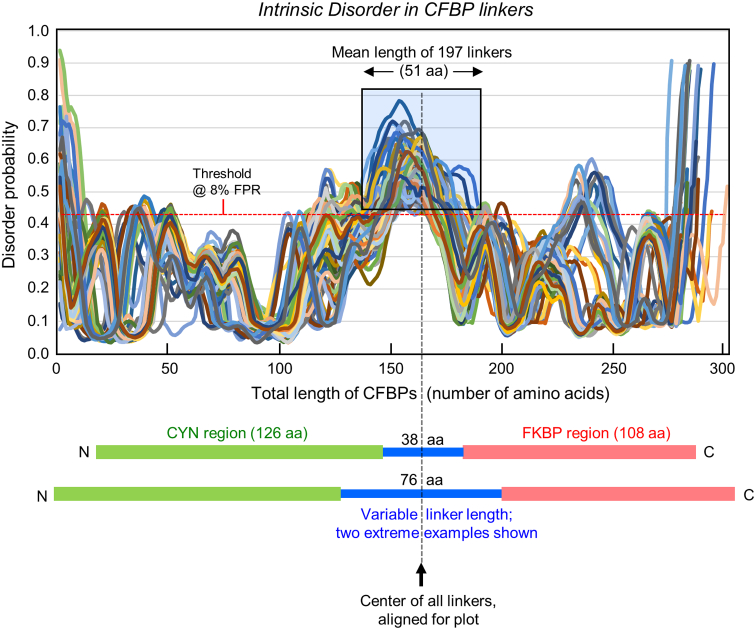
Fig. 4.
Intrinsic disorder is a common feature in all CFBP linkers. Analysis and plot of the disorder probability have been described in detail under Materials and Methods. Each CFBP graph is color-coded by the default color pattern of Excel. Amino acid number is on X-axis, and disorder probability, on Y-axis. The range of linker sequence length in the population (38 and 76 amino acids, and shortest and the longest, respectively) is illustrated schematically at the bottom (Blue in color view), along with the constant lengths of the conserved CYN (Green in color) and FKBP (Red in color) regions, chosen for this analysis. The baseline (threshold) and the FPR (False Positive Rate) have been described in Materials and Methods. Within the premise of this paper, the abnormally high disorder probabilities at the very termini of all CFBP are to be ignored, as terminal disorder is a general feature of all proteins, commonly found in X-ray crystallography [26].