Abstract
The contamination of foods and animal feeds with trichothecene mycotoxins is a growing concern for human and animal health. As such, large quantities of pure trichothecene mycotoxins are necessary for food safety monitoring and toxicological research. A new and effective method for the purification of trichothecene mycotoxins from a marine fungus, Fusarium sp. LS68, is described herein. Preparative high-speed countercurrent chromatography (HSCCC) was utilized for the scalable isolation and purification of four trichothecene mycotoxins for the first time in stepwise elution mode, with a biphasic solvent system composed of hexanes–EtOAc–CH3OH–H2O (6:4:5:5, v/v/v/v) and (8.5:1.5:5:5,v/v/v/v). This preparative HSCCC separation was performed on 200 mg of crude sample to yield four trichothecene mycotoxins, roridin E (1), roridin E acetate (2), verrucarin L acetate (3), and verrucarin J (4) in a single run, with each of >98% purity. These compounds were identified by MS, 1H NMR, 13C NMR, and polarimetry. The results demonstrate an efficient HSCCC method for the separation of trichothecene mycotoxins, which can be utilized to produce pure commercial and research standards.
Keywords: trichothecene mycotoxins, roridin, verrucarin, high-speed countercurrent chromatography, preparative separation, stepwise elution
1. Introduction
According to the Food and Agriculture Organization of the United Nations, about 25% of the food crops in the world are contaminated with mycotoxins, and these have adverse effects on humans, animals, and crops that result in serious illnesses and economic losses [1]. Among the major mycotoxins produced by Fusarium species, trichothecenes are pathogenic to important agricultural crops and foods [2], and these lead to serious economic losses by reducing yields and overall quality of North American agricultural products [3,4]. For example, Fusarium head blight disease, caused by trichothecene mycotoxins, has been shown to have contaminated cereals and grains [5,6].
Trichothecenes are a group of tetracyclic sesquiterpene mycotoxins that are produced by various fungi from the order Hypocreales, including those of the genera Fusarium, Myrothecium, Verticimonosporium, Stachybotrys, Trichoderma, Trichothecium, Cephalosporium, and Cylindrocarpon [7,8,9,10,11]. These molecules are potent phytotoxins, and act as the virulence factors of pathogenic fungi on sensitive infected host plants, particularly wheat and barley [12,13,14]. The unfortunate contamination of botanical dietary supplements with mycotoxins has also been reported recently, representing a significant risk to public health [15]. One obstacle to the further investigation of these important topics has been the scarce quantity of pure compounds from the trichothecene class. Therefore, the preparative separation of high purity trichothecene mycotoxins is of great interest. At present, trichothecene mycotoxins are isolated from fungal extracts by conventional methods, such as macroporous resin separation followed by silica gel column chromatography. These methods can be considered costly, laborious, and time-consuming, and furthermore result in substantial sample loss due to irreversible adsorption to the silica [16,17]. It is therefore important to develop rapid and efficient methods for the separation and purification of trichothecene mycotoxins.
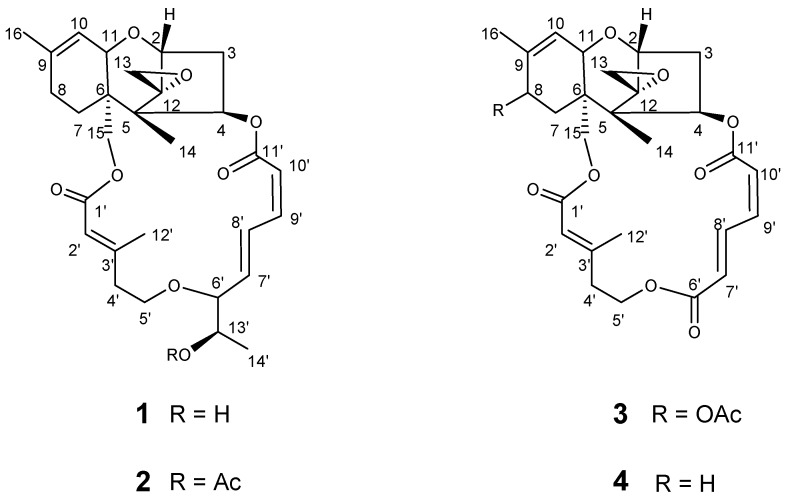
High-speed countercurrent chromatography (HSCCC) is a technique that uses liquid-liquid partition chromatography to improve recovery rates and efficiency, and which eliminates irreversible adsorption by solid support media [17,18,19]. HSCCC has been widely used for the preparative separation and purification of natural products, and this is a scalable technology [20,21]. Disclosed herein is the first preparative isolation and purification of trichothecene mycotoxins by HSCCC in stepwise elution mode, allowing for the purification of several compounds in one run. The trichothecene mycotoxins roridin E (1), roridin E acetate (2), verrucarin L acetate (3), and verrucarin J (4) were thus isolated from an epiphytic fungus, Fusarium sp. LS68, which was derived from the sponge Haliclona sp. The structures of 1–4 are presented in Figure 1.
Figure 1.
Structures of roridin E (1), roridin E acetate (2), verrucarin L acetate (3), verrucarin J (4).
2. Results and Discussion
2.1. HPLC Analysis of the Crude Extract
An HPLC method was developed to ensure the baseline separation of the target compounds and impurities. Different solvent systems, flow rates, detection wavelengths, and column temperatures were screened. The components of interest, 1–4, were satisfactorily separated by a CH3CN/H2O gradient (flow rate 0.8 mL/min, 25–75% CH3CN from 0–60 min) when the column temperature was set at 25 °C. The HPLC chromatogram of the crude extract is shown in Figure 2, Panel A. The same method was used to analyze HSCCC method development samples, and later HSCCC eluents (Figure 2, Panels B–E), for fraction pooling.
Figure 2.
Representative analytical HPLC chromatograms. (A) Crude extract of Fusarium sp; (B) refined peak 1 from HSCCC, corresponding to compound 1; (C) refined peak 2 from HSCCC, corresponding to compound 3; (D) refined peak 3 from HSCCC, corresponding to compound 2; (E) refined peak 4 from HSCCC, corresponding to compound 4. For B–D, UV profiles of the major peak are inset at 254 nm.
2.2. Optimization of Suitable HSCCC Solvent System
In order to efficiently separate and purify target compounds, the selection of an optimal biphasic solvent system is critical for preparative HSCCC. According to the “golden rules of HSCCC”, satisfactory partition coefficient KD values should meet some basic requirements: (1) the target compounds should each be in the range of 0.5 ≤ KD ≤ 2.0; (2) each set of two target compounds should have the separation factor α > 1.5, where α is the ratio of the two KD values (α = KD1/KD2, for KD1 > KD2); and (3) higher retention of the stationary phase will provide better peak resolution [22]. Thus, preliminary studies were carried out to examine the KD values of the trichothecene mycotoxins 1–4 in different hexanes–EtOAc–CH3OH–H2O solvent systems of different volume ratios by liquid-liquid partitioning and HPLC analysis. The KD values for 1–4 in the various solvent systems tested are shown in Table 1.
Table 1.
Partition coefficients (KD) of compounds 1–4 in several hexanes–EtOAc–CH3OH–H2O solvent systems tested for high-speed countercurrent chromatography (HSCCC) separation.
| Solvent System (v/v) Hexanes–EtOAc–CH3OH–H2O |
KD | |||
|---|---|---|---|---|
| Compound 1 | Compound 2 | Compound 3 | Compound 4 | |
| 1:1:1:1 | 2.08 | 2.61 | 2.33 | 10.58 |
| 5.5:4.5:5:5 | 0.93 | 2.25 | 2.14 | 4.73 |
| 6:4:5:5 | 0.61 | 1.83 | 1.26 | 3.32 |
| 6.5:3.5:5:5 | 0.37 | 1.43 | 1.12 | 2.58 |
| 7:3:5:5 | 0.45 | 1.25 | 0.80 | 3.21 |
| 7.5:2.5:5:5 | 0.36 | 1.10 | 0.68 | 2.73 |
| 8:2:5:5 | 0.19 | 0.58 | 0.54 | 1.87 |
| 8.5:1.5:5:5 | 0.11 | 0.37 | 0.32 | 1.12 |
| 9:1:5:5 | 0.10 | 0.30 | 0.25 | 0.83 |
No single suitable biphasic solvent system was found that had KD values between 0.5 and 2 for each of compounds 1–4 at the same time. To overcome this challenge, a stepwise HSCCC elution mode was chosen in order to separate compounds with significantly different KD values in a single run. This technique had been successfully applied previously by some research groups [23,24].
The use of the “HeMWat”, or hexanes–EtOAc–CH3OH–H2O, solvent system at volumetric ratios of 1:1:1:1 and 5.5:4.5:5:5 both afforded larger KD values unsuitable for the separation of the trichothecene mycotoxins investigated. However, the biphasic solvent system of hexanes–EtOAc–CH3OH–H2O at a volumetric ratio of 6.5:3.5:5:5 yielded acceptable KD values of compounds 1–3 between 0.5 and 2, although compounds 2 and 3 had an unsuitable separation factor α < 1.5. However, the biphasic hexanes–EtOAc–CH3OH–H2O solvent system at a volume ratio of 6:4:5:5 led to the observation of the KD values between 0.5 and 2 for compounds 1, 2, and 3, and each pair had a separation factor α > 1.5. Nevertheless, this solvent system offered an unsuitable KD value (2.58) for compound 4. To overcome this problem, the hexanes–EtOAc–CH3OH–H2O 8.5:1.5:5:5 solvent system was selected for the second part of the HSCCC run, since it produced a suitable KD value of 1.12 for compound 4, and it would only be used in stepwise mode after the elution of 1–3.
2.3. Stepwise HSCCC Separation
The optimized stepwise elution method was applied for the direct preparative HSCCC separation of 200 mg of crude Fusarium extract in a single run. As shown in Figure 3, the separation initiated with the solvent system of hexanes–EtOAc–CH3OH–H2O 6:4:5:5, and the upper phase was used as the stationary phase while the lower phase was used as the mobile phase in the head-to-tail elution mode. Retention of the stationary phase was 63%, and after peaks 1–3 eluted at 102, 180, and 205 min, respectively, the mobile phase was switched to the lower phase of the solvent system (8.5:1.5:5:5) at 250 min. Peak 4 was well resolved, and eluted at 350 min. Timewise fractions were collected from the HSCCC separation and pooled after evaluation by HPLC, to yield compound 1 (11.3 mg), compound 2 (7.7 mg), compound 3 (4.7 mg), and compound 4 (21.3 mg). Each compound isolated had a purity of >98% based on UV peak integrations (Figure 2, Panels B–E). The chromatogram of HSCCC separation is shown in Figure 3.
Figure 3.
HSCCC chromatogram of the crude extract from Fusarium sp. LS68 using stepwise elution with solvent systems A and B. The dotted line represents the time at which the solvent system was switched from A to B. Solvent system A: hexanes–EtOAc–CH3OH–H2O (6:4:5:5, v/v/v/v), solvent system B: hexanes–EtOAc–CH3OH–H2O (8.5:1.5:5:5, v/v/v/v); stationary phase: upper phase of solvent system A; mobile phase: lower aqueous phase of solvent system A and B; column capacity, 350 mL; rotation speed, 800 rpm; column temperature, 25 °C; flow rate, 2.0 mL/min; detection, 254 nm; sample injected, 200 mg in 6 mL biphasic solution; retention of the stationary phase, 63%; peak identification: roridin E (1), roridin E acetate (2), verrucarin L acetate (3), verrucarin J (4).
2.4. Structural Identification
The structural identification of 1–4 was conducted by HRESI-MS, 1H and 13C NMR analyses, as well as specific rotation data, and these were compared with literature values. Accordingly, the molecules isolated were identified as roridin E (1) [25], roridin E acetate (2) [26], verrucarin L acetate (3) [27], and verrucarin J (4) [27] (see Supplementary Materials).
3. Materials and Methods
3.1. Reagents and Materials
All solvents used for HSCCC were of analytical grade (Huadong Chemicals, Hangzhou, China). Reverse osmosis Milli-Q water (18 M) (Millipore, Bedford, MA, USA) was used for all solutions and dilutions. Methanol used for HPLC analyses was of chromatographic grade and purchased from Anpel Laboratory Technologies (Shanghai, China). The CDCl3 used for NMR analyses was purchased from Tenglong Weibo Technology (Qingdao, China).
3.2. Fungal Material
The marine fungi Fusarium sp. LS68, was isolated and cultured from a Halicloma sp. sponge collected from Linshui, Hainan Province, China. It was identified as Fusarium sp. according to the morphological and molecular (ITS rDNA sequence) analyses (GenBank accession number EU860057, 99% similarity). A voucher specimen was deposited at the School of Marine Sciences, Ningbo University (Ningbo, China).
3.3. Apparatus
HSCCC was carried out using a model TBE-300C high-speed countercurrent chromatograph (Tauto Biotech Co. Ltd., Shanghai, China), containing a self-balancing three-coil centrifuge rotor equipped with three preparative multilayer coils and a total capacity of 320 mL. The internal diameter of PTFE (Polytetrafluoroethylene) tubing was 1.9 mm. The apparatus maximum rotational speed is 1000 rpm and has a 20 mL manual sample loop. The revolution radius was 5 cm and the β value of the multilayer coil varied from 0.5 at the internal terminal to 0.8 at the external terminal. An integrated TBP 5002 (Tauto Biotech Co. Ltd.) was used to pump the two-phase HSCCC solvent system, and the UV absorbance of the effluent was measured at 254 nm by a UV 1001 detector (Shanghai Sanotac Scientific Instruments Co. Ltd., Shanghai, China). A DC-0506 constant temperature regulator (Tauto Biotech Co. Ltd.) was used to control the temperature during HSCCC. An N2000 data analysis system (Institute of Automation Engineering, Zhejiang University, Hangzhou, China) was employed for HSCCC data collection and analysis. The analytical HPLC equipment was an Alliance 2695 equipped with a model 2998 diode array detector and Empower System (Waters Co., Milford, MA, USA). NMR experiments were carried out using a DirectDrive2 600 MHz NMR spectrometer (Agilent, Santa Clara, CA, USA) at 25 °C and Auto Triple Resonance (Agilent, Santa Clara, CA, USA). HRESIMS data were measured using a Waters Q-TOF Premier LC/MS spectrometer (Waters Co., Milford, MA, USA). Column chromatography was conducted with silica gel (200–300 mesh, Qingdao Marine Chemical Inc. Qingdao, China). The UV spectra were recorded on an NADE Evolution 201 spectrophotometer (Waters Co., Milford, CT, USA). Optical rotations were measured on an Autopol VI (Rudolph Research Analytical, Hackettstown, NJ, USA).
3.4. Culturing and Extraction
The fungus Fusarium sp. LS68 was cultivated in a seawater-based potato dextrose broth (PDB) medium and incubated on a rotary shaker at 150 rpm and 25 °C for 14 days. The fermentation broth (30 L) was extracted with EtOAc (3 × 30 L). The organic solvent partitions were combined and evaporated under reduced pressure at 40 °C to yield a crude extract (30 g). This sample was stored in a refrigerator at 4 °C until the subsequent HSCCC separation.
3.5. Determination of Partition Coefficients (KD)
In order to determine the appropriate solvent systems for optimal partition coefficients (KD), various solvent combinations of hexanes–EtOAc–CH3OH–H2O were attempted. A 2-mg sample of crude extract was added to a 10 mL test tube along with 8 mL of an experimental biphasic solvent system. The test tube was then capped and shaken vigorously for 3 min, to allow the distribution of the extract between the two phases. After reaching equilibration, equal aliquots of each phase (10 μL) were analyzed by HPLC-DAD to determine the partition coefficient (KD) of each target compound. The KD values were defined as the integrated peak area of each target compound in the upper phase divided by that in the lower phase, as observed at 254 nm.
3.6. HSCCC Separation
The biphasic solvent systems of hexanes–EtOAc–CH3OH–H2O (6:4:5:5 and 8.5:1.5:5:5) were selected for the HSCCC separation. A 200 mg sample of crude extract was dissolved in 6 mL of each phase of a solvent mixture consisting of equal volumes of both upper and lower phases for preparative HSCCC separation. In the single run, the upper (stationary) phase was pumped into and filled the coil column of TBE-300C (320 mL, Tauto Biotech Co. Ltd., Shanghai, China) by a constant flow at 10.0 mL/min. The column was then rotated at a speed of 800 rpm, and the lower (mobile) phase was pumped into the column in the “head to tail” elution mode at 2.0 mL/min. When the mobile phase began to elute at the tail outlet, the hydrodynamic equilibrium state of the two phases was established. Subsequently, the sample solution (6 mL) containing 200 mg of crude extract was injected through the sample port. After 250 min, the pump was stopped and then the lower phase of hexanes–EtOAc–CH3OH–H2O (8.5:1.5:5:5) was pumped into the column as the new mobile phase at 2.0 mL/min. The separation temperature was controlled at 25 °C. The effluent was continuously monitored with a UV detector (Shanghai Sanotac Scientific Instruments Co. Ltd., Shanghai, China) at 254 nm. After the separation procedure, the solvents in the column were completely eluted and collected. Fractions were collected near 110 min, 180 min, 210 min, and 380 min, respectively, and analyzed by HPLC to allow for fraction pooling.
3.7. HPLC Analysis and Identification of the Peaks
Each fraction generated by preparative HSCCC was evaluated by HPLC, using a 150 mm × 4.6 mm i.d., 5 μm, YMC-Pack ODS-A column (Waters Co., Milford, CT, USA) that was maintained at 25 °C. The samples were separated with a CH3CN/H2O gradient (flow rate 0.8 mL/min, 25–75% CH3CN from 0–60 min). The effluent was continuously monitored by a UV detector at 220 nm and 254 nm. Fractions that showed only one peak in the chromatogram were respectively pooled together to yield compounds 1 (11.3 mg, the tR(HPLC) 32 min, tR(HSCCC) 102–120 min), 2 (7.7 mg, tR(HPLC) 37 min, tR(HSCCC) 180–195 min), 3 (4.7 mg, tR(HPLC) 35 min, tR(HSCCC) 205–220 min), and 4 (21.3 mg, tR(HPLC) 40 min, tR(HSCCC) 350–400 min). Compounds 1–4 were identified as roridin E (1) [25], roridin E acetate (2) [26], verrucarin L acetate (3) [27], and verrucarin J (4) [27] by comparison of their UV, 1H and 13C NMR, and specific rotation data with literature values. The purity levels of these samples were determined by peak area percentage after the automated integration of each chromatogram.
4. Conclusions
Trichothecenes are worldwide plant toxins that cause enormous yield losses in major economic crops. Some researchers have highlighted the need to decontaminate mycotoxins in foods and feeds [28]. However, investigations of trichothecene phytotoxicity are limited due to the difficulty and expense of obtaining trichothecene mycotoxins in high purity and sufficient quantity. In this study, the first efficient preparative separation of four trichothecene mycotoxins from the marine fungi Fusarium sp. LS68 by a stepwise HSCCC elution method was developed. The purities of the trichothecene mycotoxins isolated, 1–4, were >98%. Therefore, the stepwise HSCCC method is an efficient technique for the isolation of high purity trichothecene mycotoxins. This method is industrially scalable with the size of the HSCCC instrumentation, and may provide a means to obtain pure trichothecene mycotoxins in the quantities needed for broader biological examinations and the generation of analytical standards for the agricultural food, supplement, and feedstock industries.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (41776168, 41706167), the Natural Science Foundation of Zhejiang Province (LY15C200004), Scientific Research Fund of Zhejiang Provincial Education Department (Y201737271), Ningbo Sci. & Tech. Projects for Common Wealth (2015C10026, 2017C10016), the National 111 Project of China, the Li Dak Sum Yip Yio Chin Kenneth Li Marine Biopharmaceutical Development Fund, and the K.C. Wong Magna Fund in Ningbo University.
Supplementary Materials
The following are available online at http://www.mdpi.com/1660-3397/16/2/73/s1, Figure S1a, 1H NMR spectrum of compound 1 (CDCl3, 600 MHz); Figure S1b, 13C NMR spectrum of compound 1 (CDCl3, 150 MHz); Figure S1c, HRESIMS spectrum of compound 1; Figure S1d, UV spectrum of compound 1; Figure S2a, 1H NMR spectrum of compound 2 (CDCl3, 600 MHz); Figure S2b, 13C NMR spectrum of compound 2 (CDCl3, 150 MHz); Figure S2c, HRESIMS spectrum of compound 2; Figure S2d, UV spectrum of compound 2; Figure S3a, 1H NMR spectrum of compound 3 (CDCl3, 600 MHz); Figure S3b, 13CNMR spectrum of compound 3 (CDCl3, 150 MHz); Figure S3c, HRESIMS spectrum of compound 3; Figure S3d, UV spectrum of compound 3; Figure S4a, 1H NMR spectrum of compound 4 (CDCl3, 600 MHz); Figure S4b, 13CNMR spectrum of compound 4 (CDCl3, 150 MHz); Figure S4c, HRESIMS spectrum of compound 4; Figure S4d, UV spectrum of compound 4; Table S1, NMR data of compound 1 in CDCl3; Table S2, NMR data of compound 2 in CDCl3; Table S3, NMR data of compound 3 in CDCl3; Table S4, NMR data of compound 4 in CDCl3; Table S5, Specific rotation values of compounds 1–4; Table S6, UV values of compounds 1–4.
Author Contributions
Conceived and designed the experiments: Lijian Ding, Shan He. Collect the samples: Shan He, Yong Liu, Yanbin Lu. Performed the experiments: Yong Liu, Xuezhen Zhou. Analyzed the data: Yong Liu, Lijian Ding. Wrote the paper: Yong Liu, C. Benjamin Naman, Lijian Ding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Cast I. Mycotoxins: Risks in Plant, Animal, And Human Systems. Council for Agriculture Science and Technology; Ames, IA, USA: 2003. Task Force Report No. 139. [Google Scholar]
- 2.Berthiller F., Crews C., Dall’Asta C., Saeger S.D., Haesaert G., Karlovsky P., Oswald I.P., Seefelder W., Speijers G., Stroka J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013;57:165–186. doi: 10.1002/mnfr.201100764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMullen M., Jones R., Gallenberg D. Scab of wheat and barley: A re-emerging disease of devastating impact. Plant Dis. 1997;81:1340–1348. doi: 10.1094/PDIS.1997.81.12.1340. [DOI] [PubMed] [Google Scholar]
- 4.Goswami R.S., Kistler H.C. Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 2004;5:515–525. doi: 10.1111/j.1364-3703.2004.00252.x. [DOI] [PubMed] [Google Scholar]
- 5.Starkey D.E., Ward T.J., Aoki T., Gale L.R., Kistler H.C., Geiser D.M., Suga H., Tóth B., Varga J., O’Donnell K. Global molecular surveillance reveals novel fusarium head blight species and trichothecene toxin diversity. Fungal Genet. Biol. 2007;44:1191–1204. doi: 10.1016/j.fgb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Kazan K., Gardiner D.M., Manners J.M. On the trail of a cereal killer: Recent advances in Fusarium graminearum pathogenomics and host resistance. Mol. Plant Pathol. 2012;13:399–413. doi: 10.1111/j.1364-3703.2011.00762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grove J.F. Macrocyclic trichothecenes. Nat. Prod. Rep. 1993;10:429–448. doi: 10.1039/np9931000429. [DOI] [PubMed] [Google Scholar]
- 8.Grove J.F. The trichothecenes and their biosynthesis. Prog. Chem. Org. Nat. Prod. 2007;38:63–130. doi: 10.1002/chin.200715240. [DOI] [PubMed] [Google Scholar]
- 9.Wilkins K., Nielsen K.F., Din S.U. Patterns of volatile metabolites and nonvolatile trichothecenes produced by isolates of Stachybotrys, Fusarium, Trichoderma, Trichothecium and Memnoniella. Environ. Sci. Pollut. Res. 2003;10:162–166. doi: 10.1065/espr2002.05.118. [DOI] [PubMed] [Google Scholar]
- 10.Trapp S., Hohn T., McCormick S., Jarvis B. Characterization of the gene cluster for biosynthesis of macrocyclic trichothecenes in Myrothecium roridum. Mol. Gen. Genet. 1998;257:421–432. doi: 10.1007/s004380050666. [DOI] [PubMed] [Google Scholar]
- 11.Jarvis B.B., Mazzola E.P. Macrocyclic and other novel trichothecenes: Their structure, synthesis, and biological significance. Acc. Chem. Res. 1982;15:388–395. doi: 10.1021/ar00084a002. [DOI] [Google Scholar]
- 12.Desjardins A.E., Mccormick S.P., Appell M. Structure-activity relationships of trichothecene toxins in an Arabidopsis thaliana leaf assay. J. Agric. Food Chem. 2007;55:6487–6492. doi: 10.1021/jf0709193. [DOI] [PubMed] [Google Scholar]
- 13.Alexander N.J., McCormick S.P., Ziegenhorn S.L. Phytotoxicity of selected trichothecenes using Chlamydomonas reinhardtii as a model system. Nat. Toxins. 1999;7:265–269. doi: 10.1002/1522-7189(199911/12)7:6<265::AID-NT65>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Eudes F., Comeau A., Rioux S., Collin J. Phytotoxicité de huit mycotoxines associées à la fusariose de l’épi chez le blé. Can. J. Plant Pathol. 2000;22:286–292. doi: 10.1080/07060660009500477. [DOI] [Google Scholar]
- 15.Veprikova Z., Zachariasova M., Dzuman Z., Zachariasova A., Fenclova M., Slavikova P., Vaclavikova M., Mastovska K., Hengst D., Hajslova J. Mycotoxins in plant-based dietary supplements: Hidden health risk for consumers. J. Agric. Food Chem. 2015;63:6633–6643. doi: 10.1021/acs.jafc.5b02105. [DOI] [PubMed] [Google Scholar]
- 16.Cutler H.G., Jarvis B.B. Preliminary observations on the effects of macrocyclic trichothecenes on plant growth. Environ. Exp. Bot. 1985;25:115–128. doi: 10.1016/0098-8472(85)90017-6. [DOI] [Google Scholar]
- 17.Ryu S.M., Lee H.M., Song E.G., Seo Y.H., Lee J., Guo Y.Q., Kim B.S., Kim J.J., Jin S.H., Ryu K.H. Antiviral activities of trichothecenes isolated from Trichoderma albolutescens against pepper mottle virus. J. Agric. Food Chem. 2017;65:4273–4279. doi: 10.1021/acs.jafc.7b01028. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y., Zhao Y., Gu D., Ayupbek A., Huang Y., Dou J., Ito Y., Zhang T., Aisa H.A. Separation of the minor flavonols from flos gossypii by high-speed countercurrent chromatography. J. Liq. Chromatogr. Relat. Technol. 2010;33:1502–1515. doi: 10.1080/10826076.2010.489000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutherland I.A., Fisher D. Role of counter-current chromatography in the modernisation of chinese herbal medicines. J. Chromatogr. A. 2009;1216:740–753. doi: 10.1016/j.chroma.2008.11.095. [DOI] [PubMed] [Google Scholar]
- 20.Sticher O. Natural product isolation. Nat. Prod. Rep. 2008;25:517–554. doi: 10.1039/b700306b. [DOI] [PubMed] [Google Scholar]
- 21.Oka F., Oka H., Ito Y. Systematic search for suitable two-phase solvent systems for high-speed counter-current chromatography. J. Chromatogr. A. 1991;538:99–108. doi: 10.1016/S0021-9673(01)91626-7. [DOI] [PubMed] [Google Scholar]
- 22.Ito Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A. 2005;1065:145–168. doi: 10.1016/j.chroma.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 23.He S., Wang H., Yan X., Zhu P., Chen J., Yang R. Preparative isolation and purification of macrolactin antibiotics from marine bacterium Bacillus amyloliquefaciens using high-speed counter-current chromatography in stepwise elution mode. J. Chromatogr. A. 2013;1272:15–19. doi: 10.1016/j.chroma.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 24.Huang X.Y., Ignatova S., Hewitson P., Di D.L. An overview of recent progress in elution mode of counter current chromatography. Trac-Trend Anal. Chem. 2016;77:214–225. doi: 10.1016/j.trac.2015.08.006. [DOI] [Google Scholar]
- 25.Ridge C.D., Mazzola E.P., Colesb M.P., Hinkley S.F.R. Isolation and characterization of roridin E. Magn. Reson. Chem. 2016;55:337–340. doi: 10.1002/mrc.4539. [DOI] [PubMed] [Google Scholar]
- 26.Isaka M., Punya J., Lertwerawat Y., Tanticharoen M., Thebtaranonth Y. Antimalarial activity of macrocyclic trichothecenes isolated from the fungus Myrothecium verrucaria. J. Nat. Prod. 1999;62:329–331. doi: 10.1021/np980323x. [DOI] [PubMed] [Google Scholar]
- 27.Namikoshi M., Kobayashi H., Yoshimoto T., Meguro S., Akano K. Isolation and characterization of bioactive metabolites from marine-derived filamentous fungi collected from tropical and sub-tropical coral reefs. Chem. Pharm. Bull. 2000;48:1452–1457. doi: 10.1248/cpb.48.1452. [DOI] [PubMed] [Google Scholar]
- 28.Temba B.A., Sultanbawa Y., Kriticos D.J., Fox G.P., Harvey J.J., Fletcher M.T. Tools for defusing a major global food and feed safety risk: Nonbiological postharvest procedures to decontaminate mycotoxins in foods and feeds. J. Agric. Food Chem. 2016;64:8959–8972. doi: 10.1021/acs.jafc.6b03777. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.