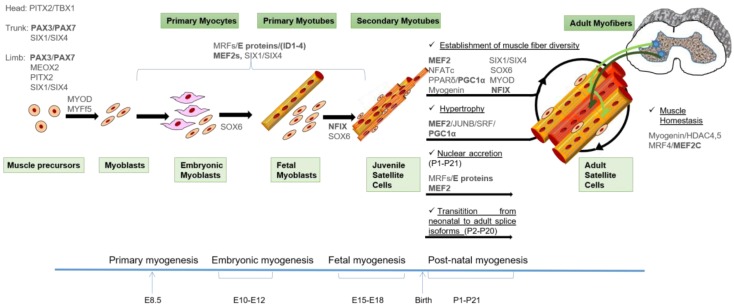
Figure 1.
Model of skeletal muscle formation and the transcription factors (TFs) involved in the control of the different waves of myogenesis. MYF5+ cells from the somitic dermomyotome are the first muscle precursors that differentiate into the myocytes of the early myotome, which provides the basic scaffold on which skeletal muscle forms in the sequential waves of myogenesis. Subsequently, PAX3/PAX7 positive cells give rise to muscle precursors during development and post-natal muscle growth: embryonic and fetal myoblasts give rise to embryonic and fetal myofibers, respectively. Satellite cells (SC) appear at the end of gestation and are responsible for postnatal growth (juvenile SC) and regeneration (adult SC). Extraocular and first branchial arch-derived myogenic progenitors are regulated by distinct gene regulatory networks where the bicoid-related Paired Like Homeodomain 2 (PITX2) and the T-box factor TBX1 TFs play a primary role. Independently of their origin, muscle differentiation of precursors depends on the activities of Muscle Regulatory Factors (MRFs) and their co-activators, the ubiquitous E proteins and the Myocyte Enhancer Factor 2 (MEF2) proteins. The fetal-specific gene expression program also involves the activity of Nuclear Factor I/X (NFIX). Post-natal muscle maturation is the result of several different processes including muscle growth (nuclear accretion and protein synthesis), fiber type specification induced by innervation and transition from embryonic to adult splicing isoforms of contractile and metabolic enzymes isoforms. The main TFs involved in the control of these processes are indicated, and the TFs that undergo alternative splicing are underlined in bold. A detailed description of the TFs networks can be found in the text. An indicative timing of murine development is depicted.