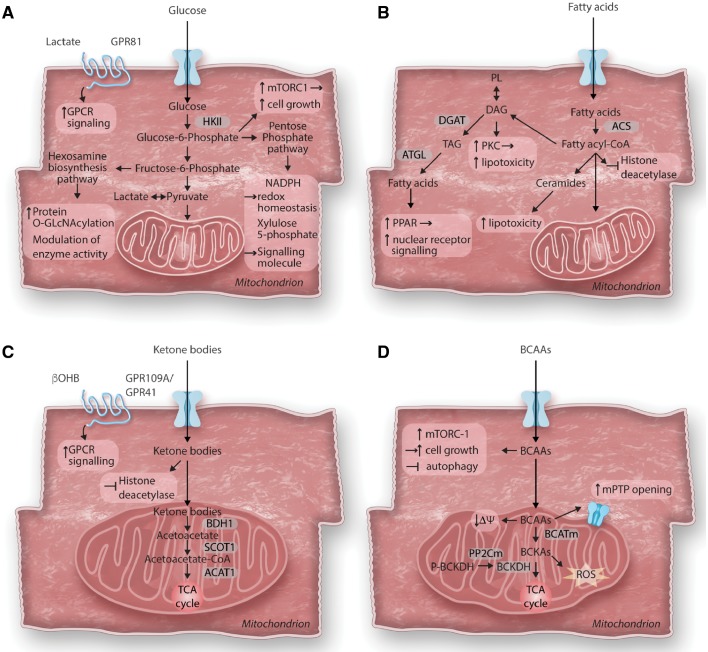
Figure 2.
Consequences of altered cardiac metabolism on non-energy providing functions. The primary role of cardiac metabolism is to provide energy for cardiac contraction. However, altered substrate utilization and metabolic remodelling in the hypertrophied heart can affect various cellular functions beyond energy supply. (A) Glucose metabolism and cardiac growth: After entering the cell, glucose is phosphorylated to glucose-6-phosphate (G6P) before feeding into glycolysis, the hexosamine biosynthesis pathway (HBP) or the pentose phosphate pathway (PPP). In cardiac hypertrophy, increased glucose reliance results in increased flux through all three pathways. Accumulation of G6P has been linked to the activation of the mechanistic target of rapamycin complex 1 (mTORC1) and cell growth. Enhanced flux through the HBP pathway results in increased protein O-GlcNAcylation and modulation of enzyme activity. Intermediates of the PPP have been recognized to act as signalling molecules (Xylulose-5-phosphate) or modulate redox homeostasis (NADPH). Recently, identification of cardiac lactate receptor (GPR81) expression proposes a role of lactate as a signalling molecule. (B) Fatty acid metabolism and cardiotoxicity: Cytosolic free fatty acids are activated by acyl CoA synthetase (ACS) to form fatty acyl-CoAs. Besides entering mitochondria for oxidation, acyl-CoAs can also form ceramides, diacylglycerol (DAG) and triacylglycerol (TAG). Formation of DAG can also occur through phospholipid breakdown which will result in protein kinase C (PKC) activation. DAG as well as ceramide species are increased in the hypertrophied heart and have been associated with lipotoxicity, while redirecting these toxic lipid intermediates into the TAG pool prevents lipotoxicity. TAG can be hydrolyzed by adipose tissue triglyceride lipase (ATGL) and can either enter the fatty acid oxidation pathway or serve as ligand for nuclear receptor activation. Acyl-CoAs have recently been identified to contribute to epigenetic regulation by acting as endogenous histone deacetylase inhibitors. (C) Ketone body metabolism and cellular signalling: Ketone bodies are oxidized in the heart for ATP generation. After entering mitochondria, they rapidly from acetyl-CoA via βOHB dehydrogenase (BDH1), succinyl-CoA:3-oxoacid-CoA transferase (SCOT) and mitochondrial acetyl-CoA acetyltransferase 1 (ACAT1). βOHB can further act as endogenous histone deacetylase inhibitor or as signalling molecule through its G protein coupled receptor (GPR109A/GPR41). (D) Branched-chain amino acids catabolism in cardiomyocyte growth and survival: Catabolism of the branched-chain amino acids (BCAA) feeds into the TCA cycle although their contribution to energy provision is rather minor under normal conditions. The first steps in BCAA catabolism yields branched-chain keto acids (BCKA) through mitochondrial branched-chain aminotransferase (BCATm) reaction. BCKA is processed by a dehydrogenase complex (BCKDH) to form CoA compounds, before further breakdown for TCA cycle. BCKDH is the rate-limiting enzyme in BCAA catabolism, it is activated by dephosphorylation through a mitochondrial-localized 2C-type protein phosphatase (PP2Cm). Accumulation of BCAA results in mitochondrial dysfunction, characterized by loss of mitochondrial membrane potential (ΔΨ) and mitochondrial permeability transition pore (mPTP) opening. BCKA accumulation stimulates ROS formation. Additionally, leucine can directly activate mTORC1 and simulate cell growth.