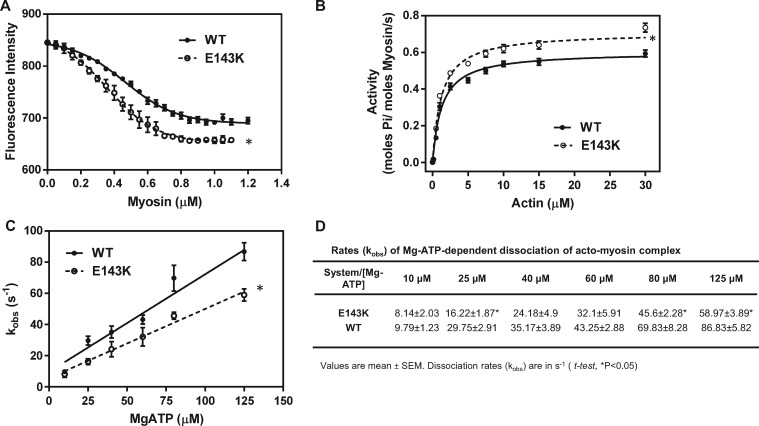
Figure 5.
Hypercontractile activity of E143K myosin. (A) Fluorescence-based binding assays of E143K or WT myosin to pyrene-labeled F-actin. Note two different profiles of binding and a higher affinity of E143K myosin (Kd = 0.368 ± 0.09 µM, n = 4 curves performed in triplicate) for pyrene-actin compared with WT (Kd = 0.465 ± 0.138 µM, n = 4). (B) Actin-activated myosin ATPase activity of E143K and WT myosins. A significantly higher Vmax was observed for E143K (0.721 ± 0.015 s−1, n = 13) vs. WT (0.615 ± 0.014 s−1, n = 11) myosin. The assays were performed in triplicate. (C) Stopped-flow assessment of kobs-[MgATP] dependence and the rates of dissociation of the acto-myosin complex in E143K vs. WT mice. The effective second order MgATP binding rates measured by the slope of ‘kobs-[MgATP]’ were: 0.444 ± 0.039 × 106 M−1 s−1, n = 4 for E143K, and 0.626 ± 0.068 × 106 M−1 s−1, n = 3 for WT, and the difference was statistically significant (t-test, P = 0.0259). (D) Dissociation rates (kobs) for individual MgATP concentrations for E143K vs. WT myosin dissociating from pyrene-labeled F-actin. Measurements were performed on myosin extracted from the left and right ventricles of 4–8 mo-old female and male E143K and WT mice. Approx. 5 hearts/per group were used to generate one batch of myosin and the experiments were repeated with 2–4 different batches of myosin. Data are presented as mean ± SEM, t-test, *P < 0.05 vs. WT.