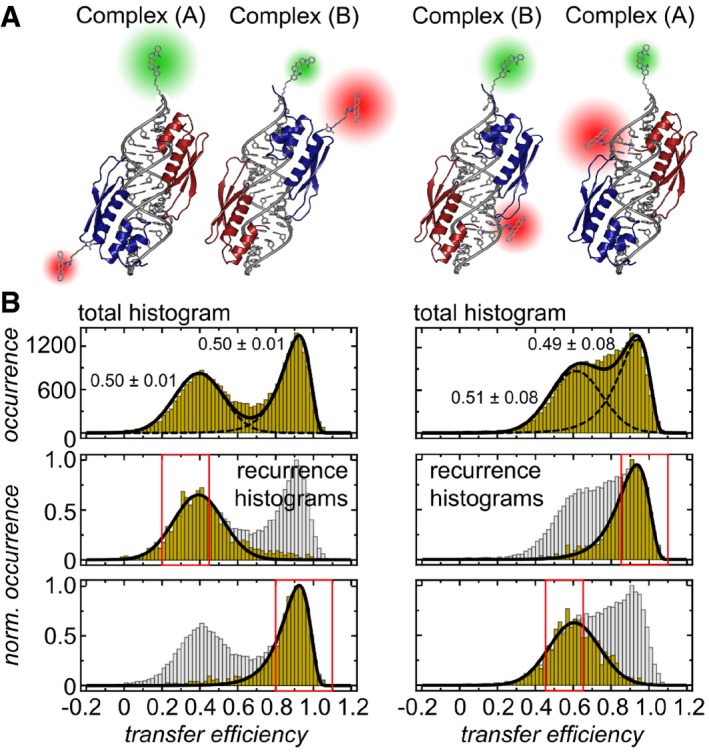
Transfer efficiency histograms of CF660R‐labeled dsRBD12_22‐235 C158S (left) and dsRBD12_22‐235 M100S (right) in complex with Cy3B‐labeled EL86. Top: Transfer efficiency histograms exhibit two subpopulations that are equally likely to occur. Errors associated with relative occurrences correspond to the standard deviation. Bottom: Recurrence transfer efficiency histograms were used to extract subpopulation‐specific fit parameters. Red boxes highlight the initial transfer efficiency range Δ
E. The recurrence interval
T was set to (0, 10 ms). See
Materials and Methods for details.