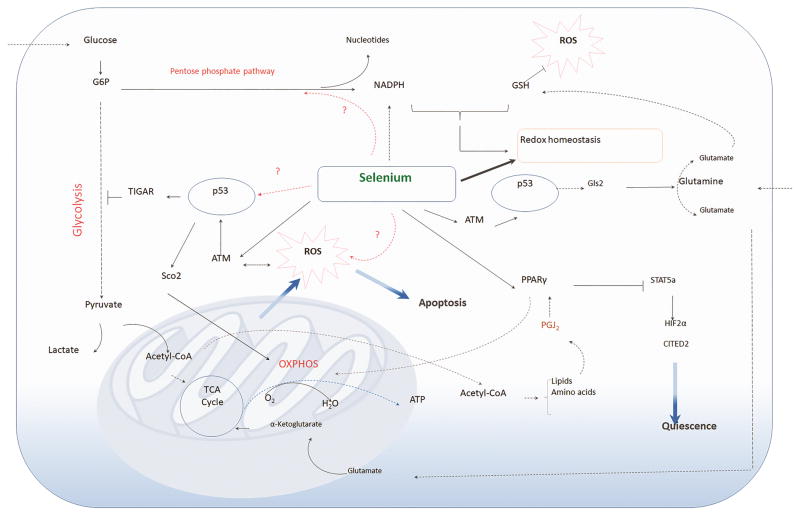
Fig. 3.
Significance of selenium in cancer stem cell metabolism. CSCs generally display glycolytic metabolic phenotype selectively coupled with TCA cycle and OXPHOS to generate energy and cellular components (amino acids and lipids) to support their stemness. Metabolically-active CSCs generate ROS, which is compensated by production of antioxidants, including GSH, NADPH derived from the pentose phosphate pathway. Selenoproteins through their redox gatekeeper functions can effectively modulate redox homeostasis. Selenium treatment of CSCs activate ATM kinase, which in turn activates p53 leading to metabolic reprogramming. p53 targets pathways via TIGAR-mediated glycolytic arrest, Sco2-mediated potentiation of OXPHOS, and Gls2-mediated regulation of glutamine metabolism to ultimately exacerbate ROS production to activate pathways of apoptosis. Selenium-mediated eicosanoid class switching leads to the production of PGJ2 as an adaptive response for protection against ROS, which is mediated through PPARγ that transcriptionally represses STAT5a to effectively inhibit downstream target genes, HIF2α and CITED2, known for maintenance of CSC quiescence, as in CML LSCs.