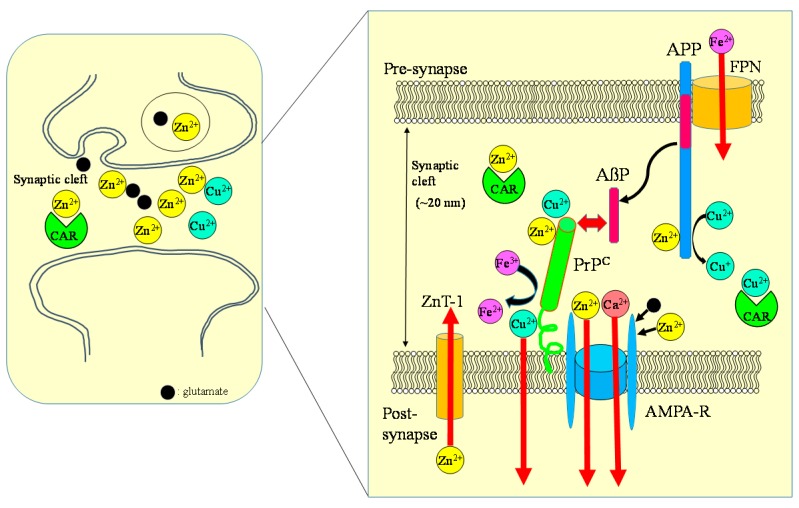
Figure 8.
Crosstalk between carnosine, metals, APP, and PrP at the synapse. Zn, Cu, and glutamate accumulate in synaptic vesicles and are released into synaptic clefts during neuronal excitation. Under normal physiological conditions, APP binds Cu and regulates Cu levels by reducing Cu2+ to Cu+. The normal prion protein isoform PrPC binds to Cu at its N-terminal domain and regulates synaptic Cu levels. It is possible that PrPC provides Cu to APP or to NMDA-type glutamate receptors, thereby influencing the production of AβP or neuronal excitability. Both APP and PrPC reportedly attenuate Cu-induced toxicity. PrPC also controls Zn2+ influx into the cells as an analogue of ZIP transporters, with AMPA-type glutamate receptors regulating synaptic Zn2+ levels. ZnT-1 is localized to postsynaptic membranes that express NMDA-type glutamate receptors and regulates Zn homeostasis. APP binds ferroportin (FPN) and regulates Fe2+ efflux. By contrast, PrPC acts as ferrireductase to regulate the Fe2+/Fe3+ ratio in synapses. The Fe2+ ions are transferred to enzymes, including neurotransmitter synthetases. Fe levels also regulate the expression of APP. It is plausible that carnosine binds to Zn2+ and Cu2+ and regulates homeostasis in these metals. ZnT-1: zinc transporter 1; NMDA-R: NMDA-type glutamate receptor; MT-3: metallothionein 3; CAR: carnosine.