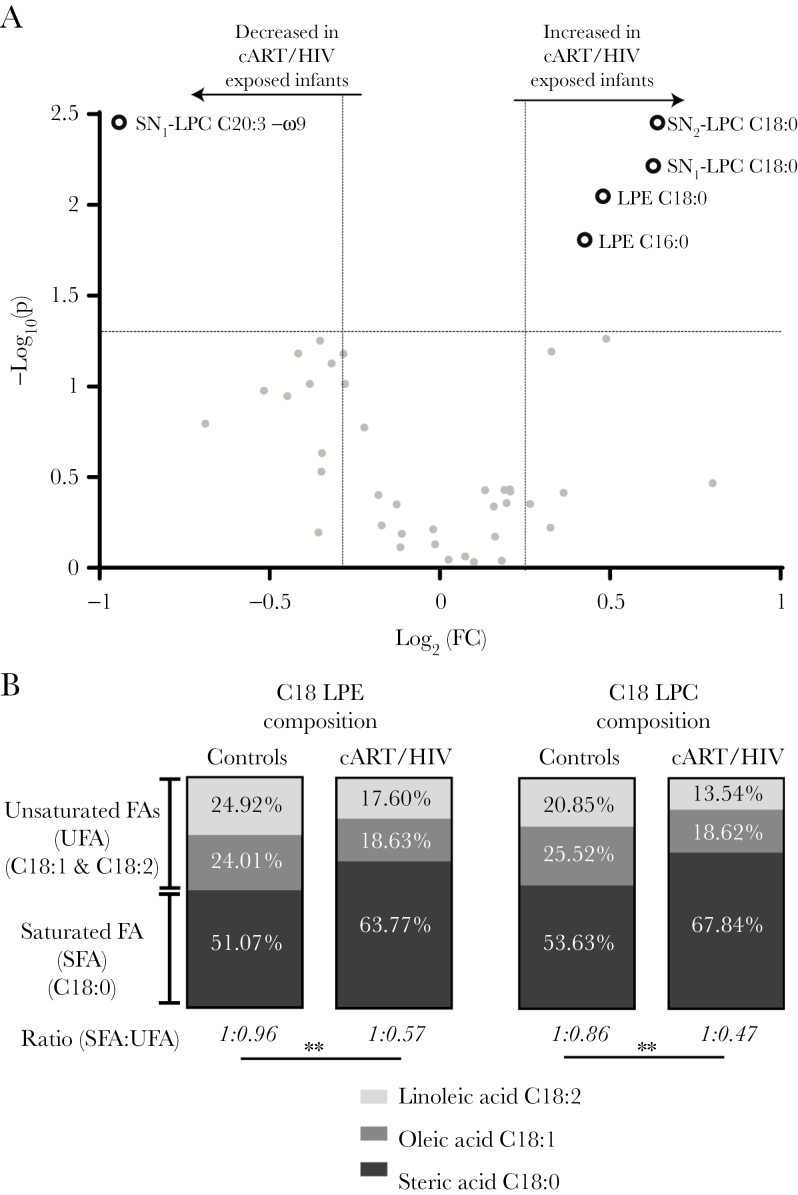
Figure 3.
The lysophospholipid profiles of combination antiretroviral therapy (cART)/ human immunodeficiency virus type 1 (HIV-1)–exposed infants vs control infants. A, Lysophospholipid profiles: The increased saturated lysophospholipids and decreased unsaturated lysophospholipid in the plasma of cART/HIV-1–exposed infants are shown as dark grey rings on the volcano plot. Volcano plots visualizes the log10 (Mann–Whitney P value) on the y-axis and the log2 (fold change [FC]) on the x-axis for each metabolite compared between the cART/HIV-1–exposed infants vs control infants. The dotted lines represent the significance thresholds with FC > 1.25 and FC < 0.75 on the x-axis and P < .05 on the y-axis. B, Lysophospholipid saturation composition average: a significant change in the ratio of saturated vs unsaturated C18 lysophosphatidylethanolamine and lysophosphatidylcholine species was found in the cART/HIV-1–exposed infants compared to control infants. Ratios revealed an almost doubling in the percentage of stearic acid vs oleic and linoleic acid species in cART/HIV-1–exposed infants. Black, gray, and light gray represents the group-averaged stearic acid (C18:0), oleic acid (C18:1), and linoleic acid (C18:2), respectively. Unpaired t test on the intergroup ratios. **P < .01. Abbreviations: cART, combination antiretroviral therapy; FA, fatty acid; FC, fold change; HIV, human immunodeficiency virus; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; SFA, saturated fatty acid; SN, stereospecific numbering; UFA, unsaturated fatty acid.