Scheme 2.
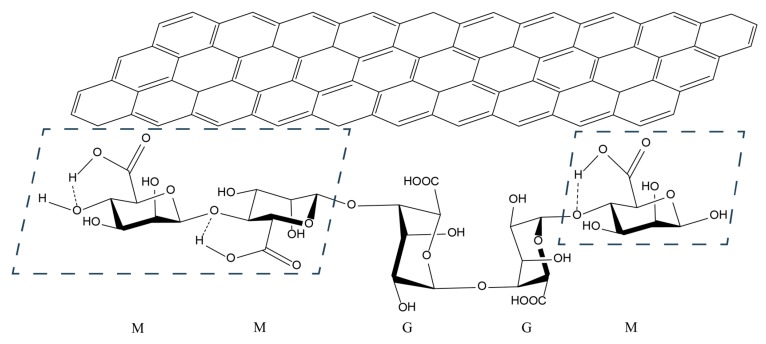
Schematic illustration of hydrophobic interactions between alginic acid (AA; bottom structure) and a graphene sheet (in carbon nanotubes and graphene; top structure). When carbon atoms of the graphene sheet are substituted with boron and nitrogen atoms, it exemplifies a boron nitride sheet (in boron nitride nanotubes and hexagonal boron nitride flakes; not shown here). The molecular structure of AA shows examples of homo-polymeric blocks of mannuronic (M) residues, homo-polymeric blocks of guluronic (G) residues, and hetero-polymeric blocks of alternating M and G residues. The ratio of M and G residues (3:2) shown here indicates the composition of AA used in this study, with ~61% mannuronic acid and ~39% guluronic acid. In M blocks, the intramolecular hydrogen bonding between carboxyl groups and glycosidic residues is favored due to the configuration of C4 and C5 [37], rendering these blocks (indicated by the blue dashed quadrilaterals) relatively hydrophobic and readily interacting with the NM hydrophobic surfaces [27]. Hydroxyl and carboxyl groups of the G blocks are unbound and oriented away from the NM surfaces, making the NM hydrophilic [27].