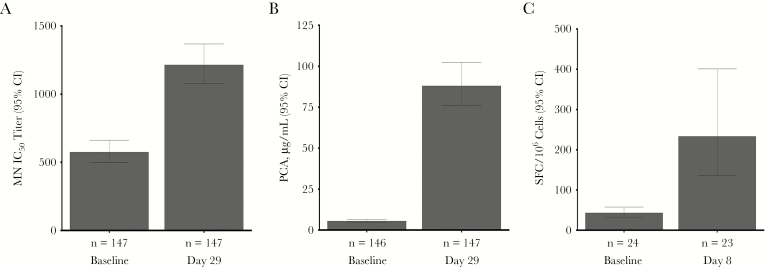
Figure 4.
Geometric mean respiratory syncytial virus (RSV) microneutralizing (MN) antibody titer (A) and palivizumab-competitive antibody (PCA) values (B) at baseline and on day 29 after dosing in subjects who met the primary end point or were selected to match them (in a 1:6 ratio) or to match the sample size of a group that received the same formulation in a phase 1b study (clinical trials registration: NCT02289820) through random sampling stratified by age and sex and controlled over baseline anti–RSV fusion protein immunoglobulin G levels, region, and comorbidity status. C, Cell-mediated response to RSV, as measured by RSV interferon γ enzyme-linked immunospot assay, in subjects who met the primary end point or who were randomly selected on the basis of sample availability. CI, confidence interval; IC50, inhibitory concentration at 50%; SFC, spot-forming cells.