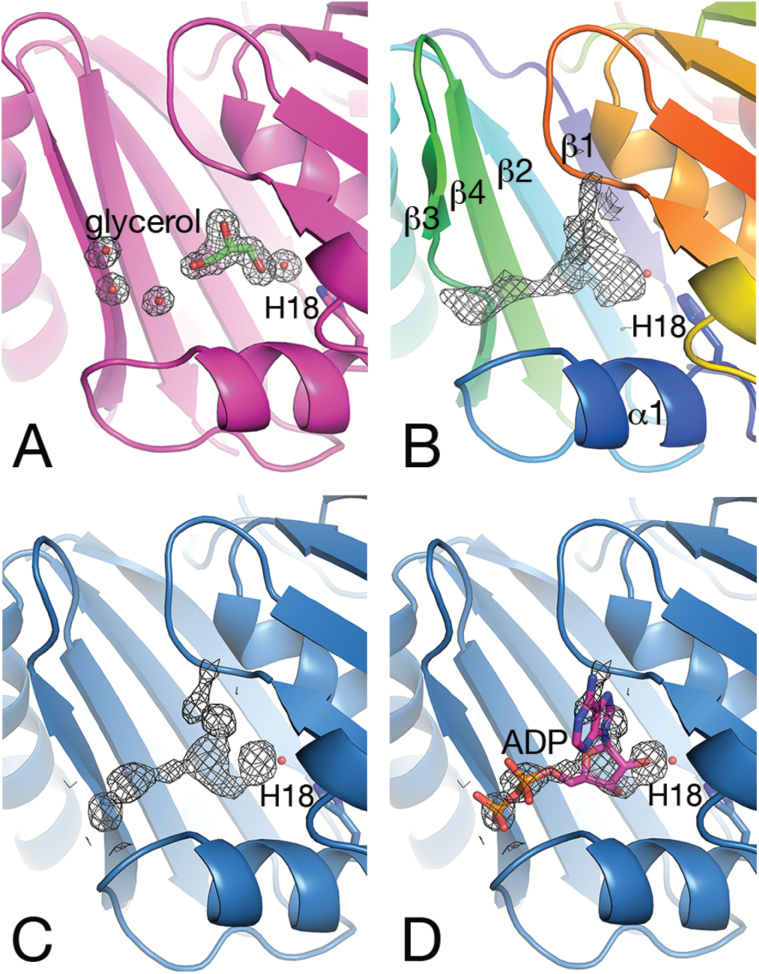
Fig. 3.
Electron density 2Fo–Fc maps in the binding pocket contoured at the 1 σ level. The side chain of His18 and the adjacent water molecule are shown as a stick representation and a red sphere, respectively. (A) Monomer A of SeCcmP_P213 (magenta) with the gating residues in an open conformation binds glycerol and a water molecule in the binding pocket between the N- and C-BMC domains. (B) Monomer B of SeCcmP_P213 (rainbow coloured from blue in the N-terminus to red in the C-terminus) with an extended electron density and the gating residues in a closed conformation. (C) Monomer A of SeCcmP_I213 (blue) with the gating residues in a closed conformation displaying a similar density as in (B). (D) ADP modelled into the density in (C).