Figure 5.
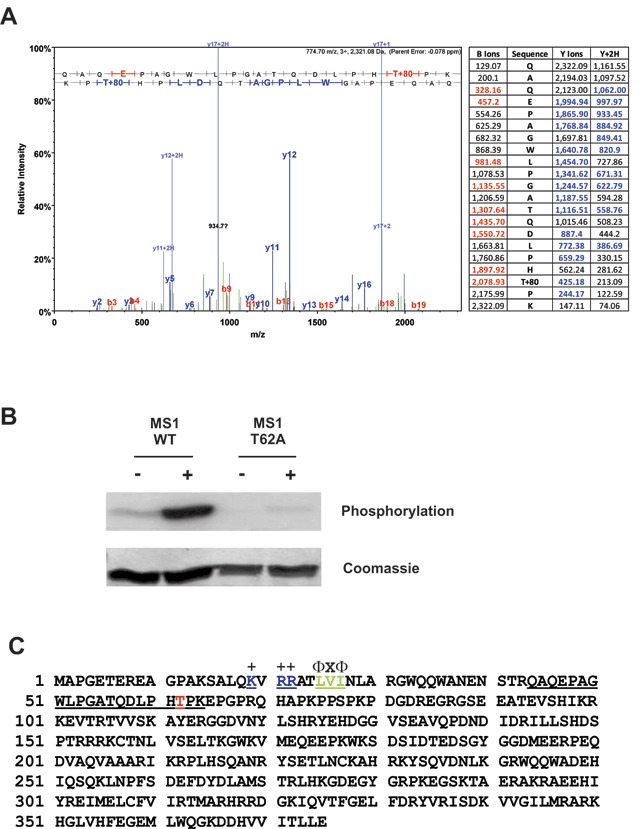
JNK phosphorylates MS1 in vitro at Threonine-62. (A) The N-terminal fragment of MS1 (1–118) was phosphorylated by JNK in vitro as described in Fig. 4A but using unlabelled ATP. The Coomassie-stained band corresponding to the MS1 substrate was excised from the gel and subjected to phosphorylation site analysis by tryptic digestion followed by LC-MS/MS. The LC-MS/MS trace and fragmentation table showing the detected b-ions (red) and y-ions (blue) are presented. (B) Active (+) or inactive (–) HA-JNK immunoprecipitated from HEK cells as described in Fig. 4A was incubated with [γ-32P] ATP and either the wild-type MS1 N-terminal fragment (1–118) or the same protein with a T62A mutation. 32P-incorporation was determined by SDS-PAGE and PhosphorImager analysis. (C) MS1 amino acid sequence showing the tryptic peptide (residues 44–64, underlined) containing Thr-62 (red) and the location of a putative MAPK docking site (residues 19–27, underlined with consensus sequence above).
