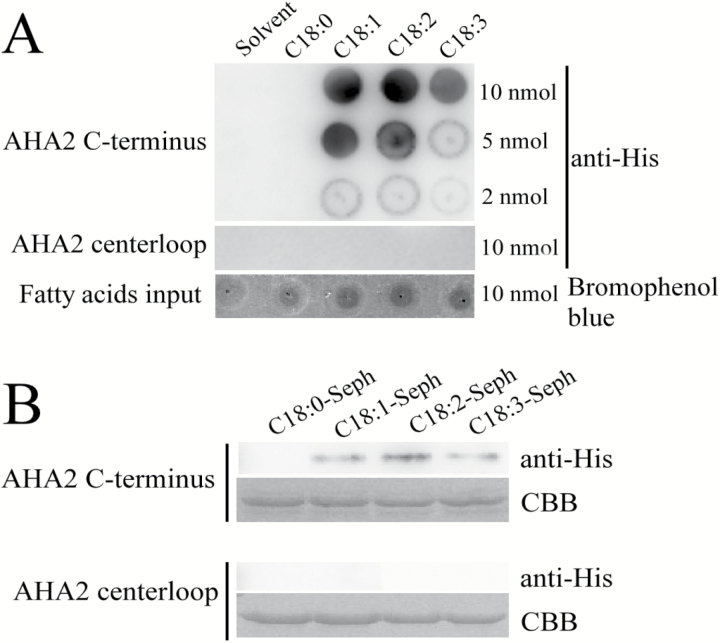
Fig. 4.
C18:1, C18:2, and C18:3 bind to the C-terminus of PM H+-ATPase AHA2. (A) Lipid–protein overlay experiment between AHA2 peptides (C-terminus and centerloop) as His fusion proteins extracted from E. coli and C18:1, C18:2, or C18:3. The solvent (DCM:methanol, 1:1) and C18:0 were used as controls. The amount that each lipid spot contained is shown on the right side. The upper lane shows the interaction between the AHA2 C-terminus and the fatty acids. The middle lane shows the interaction between the AHA2 centerloop and the fatty acids. The lower lane shows the fatty acid input control, which was stained by bromophenol blue. The experiment was repeated three times with similar results. (B) C18:0, C18:1, C18:2, and C18:3 agarose affinity chromatography experiment performed with the AHA2 peptides (C-terminus and centerloop) as His fusion proteins extracted from E. coli. The fatty acid–Sepharose matrixes were incubated with the peptides (C-terminus and centerloop) and washed with PBS, and the bound peptides were eluted and detected by immunoblot using anti-His antibodies. The upper lanes show detection using the C-terminus and the lower lanes show detection using the centerloop. The input control of each experiment was detected with Coomassie brilliant blue staining (CBB). The experiment was repeated three times with similar results.