Figure 3.
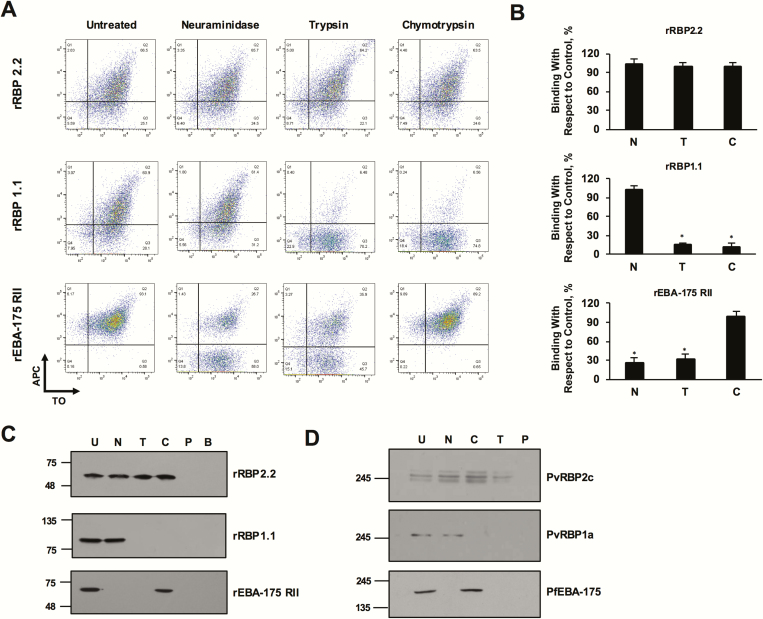
Reticulocyte-binding specificity of rRBP2.2 and rRBP1.1 recombinant proteins. A, plots depicting rRBP2.2-binding reticulocytes in a neuraminidase-, chymotrypsin-, and trypsin-resistant manner. rRBP1.1 binding with reticulocytes (thiazole orange [TO] positive) was neuraminidase (N) resistant but sensitive to chymotrypsin (C) and trypsin (T). rEBA-175RII (recombinant receptor–binding domain of PfEBA-175) was analyzed as a control with the same set of enzymatically treated reticulocytes. rPfEBA-175RII bound untreated and chymotrypsin-treated cells but had reduced binding to the neuraminidase- and trypsin-treated cells. APC, allophycocyanin. B, Bar charts showing the percentage reticulocyte binding of rRBP2.2, rRBP21.1, and rEBA-175RII. Error bars represent standard errors of the mean for 3 independent repeats. *P < .001. C, Immunoblots depicting the binding of rRBP2.2, rRBP1.1, and rPfEBA-175RII to enzyme-treated reticulocytes. No protein was detected when cells were incubated with phosphate-buffered saline or when the magnetic beads were incubated with the proteins. B, CD71 magnetic beads control; U, untreated; C, chymotrypsin treated; N, neuraminidase treated; trypsin, P, phosphate-buffered saline control; T, trypsin treated. D, Immunoblots depicting the binding of native Plasmodium vivax reticulocyte-binding protein (PvRBP) 2c, PvRBP1a, and PfEBA-175 with the enzyme-treated reticulocytes. Reticulocytes were enriched by magnetic sorting. Abbreviations: PfEBA-175, Plasmodium falciparum erythrocyte binding antigen 175; rRBP, recombinant RBP.