Table 1.
Selected Devices for Intradermal Administration of Vaccines
| Device (supplier) | Description | Registered for use | Doses per viala | Availability of clinical data for IPV | Availability of clinical data for other vaccines | |
|---|---|---|---|---|---|---|
|
ID Adapter (West Pharmaceutical Services) | Plastic adapter that fits onto an autodisable intradermal needle and syringe that is provided with the device | Yes | 5 | Yes | No |

|
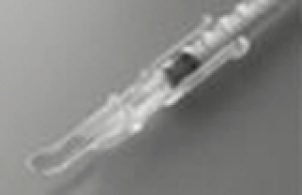
Star ID Syringe (Star) | Needle/syringe with a short minineedle and 90-degree injection angle, filled with an integrated plastic spike | No | 5 | Yes (prototype) |
No |

|
MicronJet 600 (NanoPass) | Hollow microneedle hub that can be attached to a luer syringe following filling with a separate needle | Yes | 3 | Yes | Yes |

|
Tropis (PharmaJet) | Needle-free jet injector that uses a sterile single-dose syringe and pressurized liquid stream instead of needle | Yes | 5 | Yes | Yes |

|
ID Pen (Bioject) | Needle-free jet injector developed as an alternative to Bioject’s gas-powered Biojector2000 device that is optimized for intradermal administration in low-resource settings (manually powered, intradermal only) | No | 4 | Yes | Yes |
Abbreviations: ID, intradermal; IPV, inactivated poliovirus vaccine.
aNumber of 0.1-mL doses obtained from each device from a model 0.5-mL vial [39].
