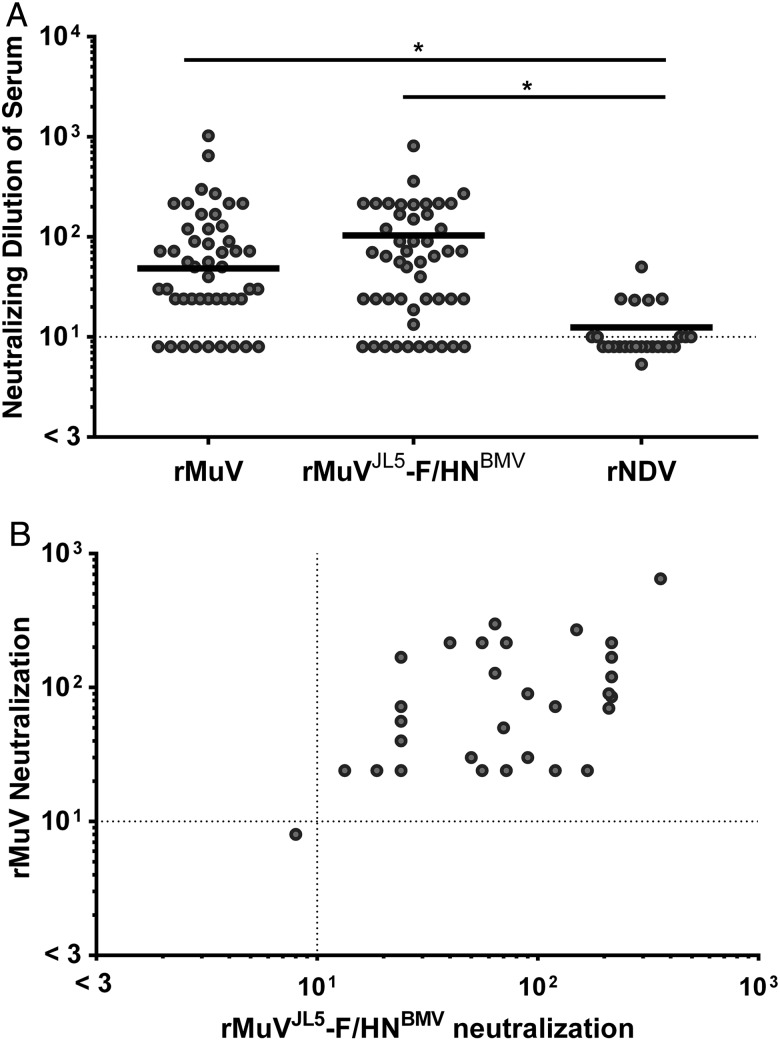
Figure 2.
Neutralization activity in a panel of human serum samples. Each data point represents the average neutralizing dilution of serum from 3 experimental replicates (error bars not shown for the purpose of clarity in the figure). A, Bold horizontal lines indicate the geometric mean neutralization values of each data set; differences between mean neutralization values were evaluated by a 1-way analysis of variance. * P < .001. The inhibitory dilution was determined as the lowest dilution in each series with <50% infection. Dashed lines represent the threshold of seropositivity. A threshold inhibitory dilution of 1:10 was used as a cutoff for seropositivity, based on several factors: (1) the limit of detection of our assay was 1:8, (2) apparent nonspecific inhibition was observed at serum concentrations >1:10, and (3) the vast majority of serum samples had an inhibitory dilution <1:10 for the negative control group recombinant Newcastle disease virus (rNDV). B, Pearson's correlation was used to determine the significance of the cross-neutralizing activity (Pearson r = 0.5793; P < .001). Dashed lines represent the threshold of seropositivity as defined above. Serum samples that were able to neutralize rNDV in addition to recombinant parental virus (rMuV) and recombinant MuV bearing the F and HN glycoproteins of BMV (rMuVJL5-F/HNBMV) were considered cytotoxic or nonspecific (5 of 59 serum samples), and as such were removed from further analyses.