Abstract
Objective. The aim was to assess the efficacy of rituximab for the cutaneous manifestations of adult DM and JDM.
Methods. Patients with refractory adult DM (n = 72) and JDM (n = 48) were treated with rituximab in a randomized placebo-phase-controlled trial [either rituximab early drug (week 0/1) or rituximab late arms (week 8/9), such that all subjects received study drug]. Stable concomitant therapy was allowed. Cutaneous disease activity was assessed using the Myositis Disease Activity Assessment Tool, which grades cutaneous disease activity on a visual analog scale. A myositis damage assessment tool, termed the Myositis Damage Index, was used to assess cutaneous damage. Improvement post-rituximab was evaluated in individual rashes as well as in cutaneous disease activity and damage scores. The χ2 test, Student’s paired t-test and Wilcoxon test were used for analysis.
Results. There were significant improvements in cutaneous disease activity from baseline to the end of the trial after rituximab administration in both adult DM and JDM subsets. The cutaneous visual analog scale activity improved in adult DM (3.22–1.72, P = 0.0002) and JDM (3.26–1.56, P <0.0001), with erythroderma, erythematous rashes without secondary changes of ulceration or necrosis, heliotrope, Gottron sign and papules improving most significantly. Adult DM subjects receiving rituximab earlier in the trial demonstrated a trend for faster cutaneous response (20% relative improvement from baseline) compared with those receiving B cell depletion later (P = 0.052).
Conclusion. Refractory skin rashes in adult DM and JDM showed improvement after the addition of rituximab to the standard therapy in a clinical trial.
Keywords: rituximab, B cell depletion, dermatomyositis, juvenile dermatomyositis, cutaneous
Rheumatology key messages
Various refractory cutaneous rashes of DM and JDM improved in frequency as well as severity after rituximab.
The cutaneous disease activity of refractory DM and JDM improved after rituximab.
Introduction
The idiopathic inflammatory myopathies are a group of acquired, heterogeneous, systemic CTDs that include adult DM and PM and JDM. Currently, there are no US Food and Drug Administration-approved therapies based on randomized controlled trials. Rituximab, a B cell-depleting agent, has been increasingly used in autoimmune diseases, given the crucial role of B cells in the initiation and propagation of the immune response. Likewise, many retrospective case reports and series have evaluated the use of rituximab in myositis in the past 10 years, including the treatment of the cutaneous features of DM [1–5]. One open-label, uncontrolled trial of seven DM patients suggested that both skin and muscle disease improved in adult DM [4], whereas another open-label uncontrolled trial of eight adult DM patients failed to show a response in severe skin disease after rituximab treatment [6]. Two patients with JDM improved in a case report, and a single-centre retrospective review of JDM patients noted that 75% (9/12) had improvement in cutaneous or muscular manifestations [1, 7]. The Rituximab in Myositis (RIM) Trial allowed the opportunity to evaluate the safety and efficacy of rituximab in treating the rash of DM in both adults and children in a prospective fashion, where the features of myositis were assessed using a validated measure of disease activity [8].
Methods
Study subjects
The RIM Trial included 200 subjects with refractory myositis (76 PM, 76 adult DM and 48 JDM), who were randomized to receive rituximab early (drug at weeks 0/1, placebo at weeks 8/9) or rituximab late arms (placebo at weeks 0/1, drug at weeks 8/9), such that all subjects received study drug. We used 72 of the 76 adult DM and 48 JDM patients for analysis in this study. They were followed for 44 weeks (14 visits), and cutaneous disease activity was assessed at several time points: week 0, 4, 8, 12, 16, 20, 24, 38, 32, 36 and 44. Rituximab dosing was based on the patient’s body surface area; children with a body surface area ⩽1.5 m2 received 575 mg/m2 at each infusion, and adults and children with a body surface area >1.5 m2 received 750 mg/m2 up to 1 g/infusion. The glucocorticoid dose was held constant, without reduction, until week 16, and i.v. glucocorticoids were not allowed at the time of any study medication infusion. Further details of the RIM Trial are published [8]. University of Pittsburgh Institutional Review Board approval and patient consents were obtained for the study.
Cutaneous disease activity assessment
At all 14 visits, cutaneous clinical disease activity was assessed using the Myositis Disease Activity Assessment Tool (MDAAT) [9]. The MDAAT is a reliable and validated outcome measure in myositis, which has been used in multiple clinical trials [9–14]. It includes a physician assessment of various cutaneous rashes of adult DM and JDM as well as the grading of cutaneous disease activity measure on a 10 cm visual analog scale (VAS). The following variables were assessed longitudinally on all RIM Trial subjects using the MDAAT: cutaneous ulceration, erythroderma, panniculitis, erythematous rashes with or without secondary changes of erosions, necrosis or vesiculobullous lesions, heliotrope rash, Gottron papules or sign, periungal capillary changes, alopecia (diffuse or focal, patchy with erythema) and mechanics hands. The grading of each of these DM-associated cutaneous clinical features at each trial visit was reported as follows: NA indicates cannot be assessed; 0 indicates not present in the last 4 weeks; 1 indicates improvement in the previous 4 weeks; 2 indicates the rash as unchanged in the previous 4 weeks; 3 refers to worsening over the last 4 weeks; and 4 indicates the new onset of that specific feature in the last 4 weeks. In addition, the global cutaneous disease activity over the past 4 weeks was assessed using the 10 cm VAS at each visit, with a score of 0 indicating no active cutaneous disease and 10 being severe active cutaneous disease. All investigators in the RIM Trial received specialized training along with a glossary and key on assessing cutaneous and other organ system involvement using the MDAAT [15].
Cutaneous damage assessment
A validated myositis damage assessment tool, termed the Myositis Damage Index (MDI), was also assessed in the RIM Trial at weeks 0 and 44 [10, 11, 13]. The MDI variables that were assessed included calcinosis, alopecia (due to damage), cutaneous scarring or atrophy, poikiloderma and lipodystrophy. Each of these cutaneous damage variables above was assessed as follows: NA indicates cannot be assessed; 0 indicates the item has never been present; and 1 indicates the presence of one of the aforementioned damage variables that has been present for at least 6 months. Similar to the MDAAT, the MDI also assessed overall cutaneous damage using a 10 cm VAS, with 0 indicating no evidence of cutaneous damage and 10 noting severe evidence of cutaneous damage according to the examining physician.
Statistical analysis
Descriptive statistics were performed on subject demographics, key clinical features and baseline cutaneous features of disease activity and damage in the adult DM, JDM and combined cohorts. The MDAAT and MDI variables were analysed for adult DM and JDM separately and for the combined cohort. Changes in the frequency of various cutaneous variables of disease activity and damage were assessed at the last visit after rituximab and compared with the baseline (pre-rituximab) finding using the χ2 test (or Fisher’s exact test). In addition, the VAS score of cutaneous disease activity and damage was assessed at the last trial visit after rituximab and likewise compared with the baseline (pre-rituximab) finding using Student’s paired t-test (before and after). The baseline for all of the above analyses was considered as week 0 for the rituximab early subset and week 8 for the rituximab late subjects, such that the findings were determined as the weeks post-rituximab (i.e. rituximab early: weeks 0, 4, 8, 12, 16, 20, 24, 28, 32, 36 and 44; and rituximab late: weeks 0, 4, 8, 12, 16, 20, 24, 28 and 36). Given that only half the patient data were available for weeks 32 and 44 (for rituximab early), these data were not included in the primary analysis. For the MDI variables, week 44 data were compared with week 0 (baseline) for all patients. In addition, a comparison of the early vs late rituximab treatment groups for time to 20, 40 and 60% relative improvement of cutaneous disease activity (10 cm VAS) by Kaplan–Meier and Wilcoxon test was determined.
Results
Baseline characteristics of JDM and DM subjects
The characteristics of the full cohort of RIM Trial subjects have been described previously [8]. The clinical characteristics of the adult DM and JDM patients are specifically listed in Table 1. In summary, most patients were female (70%), Caucasian (73%), had active cutaneous disease [mean (s.d.) baseline physician global cutaneous disease VAS: 3.2 (2.3)] and were refractory, with chronic disease [failed mean of 2.6 immunosuppressive agents and glucocorticoids; and mean (s.d.) disease duration was 5.3 (6.9) years]. Concomitant therapies included 68% MTX, 18% AZA, 7% IVIG, 9% mycophenolate and 3% tacrolimus. The most common myositis-specific autoantibodies in the adult DM patients included anti-Mi-2 (28%) and anti-synthetase (21%), whereas the JDM subjects possessed anti-NXP2 (33%) and anti-TIF1-γ (27%) autoantibodies. The baseline cutaneous rashes in the adult DM and JDM cohort are described in Table 2.
Table 1.
Summary of baseline clinical characteristics of DM, JDM and combined JDM and DM cohorts
| Baseline characteristics | DM, n = 72 | JDM, n = 48 | Combined DM and JDM, n = 120 | |||
|---|---|---|---|---|---|---|
| Caucasian, n (%) | 54 (75) | 33 (69) | 87 (73) | |||
| Age, mean (s.d.), years | 49.9 (10.4) | 15.3 (9.5) | 36.1 (19.7) | |||
| Female, n (%) | 53 (74) | 31 (65) | 84 (70) | |||
| Disease duration, mean (s.d.), years | 4.3 (4.2) | 6.8 (9.6) | 5.3 (6.9) | |||
| Prednisone dose (mg), mean (s.d.) | ||||||
| Start | 20.8 (11.7) | 17.3 (13.9) | 19.4 (12.7) | |||
| End | 16.6 (11.8) | 12.6 (10.6) | 15.0 (11.5) | |||
| MTX dose (mg), mean (s.d.) | ||||||
| Start | 20.0 (4.8) | 21.4 (6.1) | 20.6 (5.9) | |||
| End | 19.6 (5.6) | 21.7 (6.0) | 20.7 (5.5) | |||
| Failed immunosuppressive agents, mean (s.d.), n | 2.69 (1.33) | 2.58 (1.16) | 2.65 (1.26) | |||
| Concomitant immunosuppressive therapy, n (%) | Start | End | Start | End | Start | End |
| Prednisone | 55 (76) | 54 (75) | 36 (75) | 36 (75) | 91 (76) | 90 (75) |
| MTX | 43 (60) | 36 (50) | 38 (79) | 38 (79) | 81 (68) | 74 (62) |
| AZA | 16 (22) | 14 (20) | 5 (10) | 4 (8) | 21 (18) | 18 (15) |
| IVIG | 0 (0) | 2 (3) | 8 (17) | 11(23) | 8 (7) | 13 (11) |
| Mycophenolate | 8 (11) | 9 (12.5) | 3 (6) | 3 (6) | 11 (9) | 12 (10) |
| Tacrolimus | 2 (3) | 2 (3) | 1 (2) | 1 (2) | 3 (3) | 3 (3) |
| CYC | 2 (3) | 1 (1) | 0 (0) | 0 (0) | 2 (2) | 1 (1) |
| Other immunosuppressive | 4 (6) | – | 9 (19) | – | 13 (11) | 13 (11) |
| Myositis-associated antibodies, n (%) | ||||||
| Anti-synthetase | 15 (21) | 1 (2) | 16 (13) | |||
| Anti-Mi-2 | 20 (28) | 5 (10) | 25 (21) | |||
| Anti-NXP2 | 4 (6) | 16 (33) | 20 (17) | |||
| Anti-TIF1-γ | 8 (11) | 13 (27) | 21 (18) | |||
| Anti-SRP | 3 (4) | 0 (0) | 3 (3) | |||
| Others | 7 (10) | 3 (6) | 10 (8) | |||
| None | 10 (14) | 7 (15) | 17 (14) | |||
| Undefined | 5 (7) | 3 (6) | 8 (7) | |||
| Cutaneous disease activity on VAS, mean (s.d.) | 3.2 (2.5) | 3.3 (2.0) | 3.2 (2.3) | |||
| Cutaneous disease damage on VAS, mean (s.d.) | 1.5 (2.0) | 2.1 (2.3) | 1.8 (2.1) | |||
Other autoantibodies in the two different diseases include the following: adult DM: five anti-PM-Scl and two anti-U1/U2; and JDM: two anti-U1RNP and one anti-SAE antibody. VAS: visual analog scale.
Table 2.
Baseline cutaneous rashes in DM, JDM and combined DM and JDM cohorts
| DM | JDM | DM and JDM combined | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cutaneous variables | Baseline, n = 72 | Last visit, n = 67 | P- value | Baseline, n = 48 | Last visit, n = 44 | P- value | Baseline, n = 120 | Last visit, n = 111 | P- value |
| MDAAT variables | |||||||||
| Cutaneous ulcerations | 9.7 (7) | 4.5 (3) | 0.23 | 20.8 (10) | 4.5 (2) | 0.02 | 14.2 (17) | 7.2 (8) | 0.089 |
| Erythroderma | 22.2 (16) | 4.5 (3) | 0.002 | 39.5 (19) | 11.4 (5) | 0.002 | 29.2 (35) | 9.9 (11) | <0.001 |
| Panniculitis | 8.3 (6) | 2.9 (2) | 0.17 | 10.4 (5) | 6.8 (3) | 0.78 | 9.2 (11) | 7.2 (8) | 0.58 |
| Erythematous rashes | |||||||||
| With secondary change | 9.7 (7) | 4.5 (3) | 0.23 | 31.3 (15) | 22.7 (10) | 0.36 | 18.3 (22) | 9.9 (11) | 0.07 |
| Without secondary changes | 69.4 (44) | 23.9 (16) | <0.001 | 83.3 (40) | 11.4 (5) | <0.001 | 75 (90) | 54.9 (61) | 0.001 |
| Heliotrope rash | 56.9 (41) | 20.9 (14) | <0.001 | 68.8 (33) | 54.5 (5) | <0.001 | 61.7 (74) | 37.8 (42) | <0.001 |
| Gottron’s papules/sign | 5.9 (41) | 22.4 (15) | <0.001 | 91.6 (44) | 52.3 (23) | <0.001 | 70.9 (85) | 45.9 (51) | <0.001 |
| Periungal capillary changes | 61.1 (44) | 23.9 (16) | <0.001 | 77.1 (37) | 52.3 (23) | 0.012 | 67.5 (81) | 53.2 (59) | 0.026 |
| Alopecia | |||||||||
| Diffuse hair loss | 36.1 (26) | 19.4 (13) | 0.028 | 25 (12) | 18.2 (8) | 0.21 | 31.6 (38) | 28.8 (32) | 0.64 |
| Focal, patchy with erythema | 15.3 (11) | 6 (4) | 0.07 | 10.4 (5) | 0 | 0.02 | 31.6 (38) | 4.5 (5) | <0.001 |
| Mechanics hands | 30.5 (22) | 11.9 (8) | 0.008 | 10.4 (5) | 4.5 (2) | 0.29 | 22.5 (27) | 16.2 (18) | 0.23 |
| Any DM rash | 88.8 (64) | 37.3 (25) | 0.047 | 100 (48) | 75 (33) | 0.002 | 94.2 (113) | 80.2 (89) | 0.001 |
| Classic DM rash | 69.4 (50) | 26.9 (18) | 0.009 | 95.8 (46) | 59.1 (26) | <0.001 | 80 (96) | 54.0 (60) | <0.001 |
| Classic DM rash + erythematous rashes | 79.2 (57) | 31.3 (21) | 0.032 | 97.9 (47) | 72.7 (32) | <0.001 | 86.6 (104) | 70.3 (78) | <0.001 |
| MDI variables | |||||||||
| Calcinosis | 9.8 (7)a | 15.3 (10)a | 0.25 | 45.8 (22) | 56.8 (25) | 0.29 | 24.3 (29)a | 32.1 (35)a | 0.19 |
| Alopecia (damage) | 40.8 (29)a | 35.3 (23)a | 0.51 | 22.9 (11) | 25 (11) | 0.82 | 33.6 (40)a | 31.2 (34)a | 0.69 |
| Cutaneous scarring or atrophy | 21.1 (15)a | 20 (13)a | 0.19 | 52.0 (25) | 38.6 (17) | 0.19 | 33.6 (40)a | 27.5 (30)a | 0.32 |
| Poikiloderma | 9.8 (7)a | 9.2 (6)a | 0.9 | 22.9 (11) | 20.4 (9) | 0.77 | 15.1 (18)a | 13.8 (15)a | 0.77 |
| Lipodystrophy | 1.4 (1)a | 1.5 (1)a | 0.95 | 12.5 (6) | 15.9 (7) | 0.64 | 5.9 (7)a | 7.3 (8)a | 0.65 |
All values are given as n (%).
One patient did not complete the MDI form. MDAAT: Myositis Disease Activity Assessment Tool; MDI: Mysositis Damage Index.
Adult DM
Baseline disease activity and damage in adult DM
Among adult DM patients, most had an active rash 89% (64/72), which included the classic DM rashes of Gottron changes and/or a heliotrope (50/72, 69%). The baseline mean (s.d.) cutaneous disease activity was 3.2 (2.46) on a 10 cm VAS, with the most common rashes including erythematous rashes without chronic changes of erosion or necrosis (44/72, 61%) and periungual capillary changes (44/72, 61%), followed by heliotrope rash and Gottron papules (41/72, 57% and 41/72, 57%, respectively). Further details of the frequency of specific DM rashes at baseline and follow-up are noted in Table 2. The baseline mean (s.d.) cutaneous damage (10 cm VAS) was 1.5 (2.02), with 10% of patients having calcinosis, 41% of patients with alopecia, 21% with cutaneous scarring or atrophy, 10% with poikiloderma and 1.4% with lipodystrophy.
Improvement in cutaneous rashes in adult DM post-rituximab
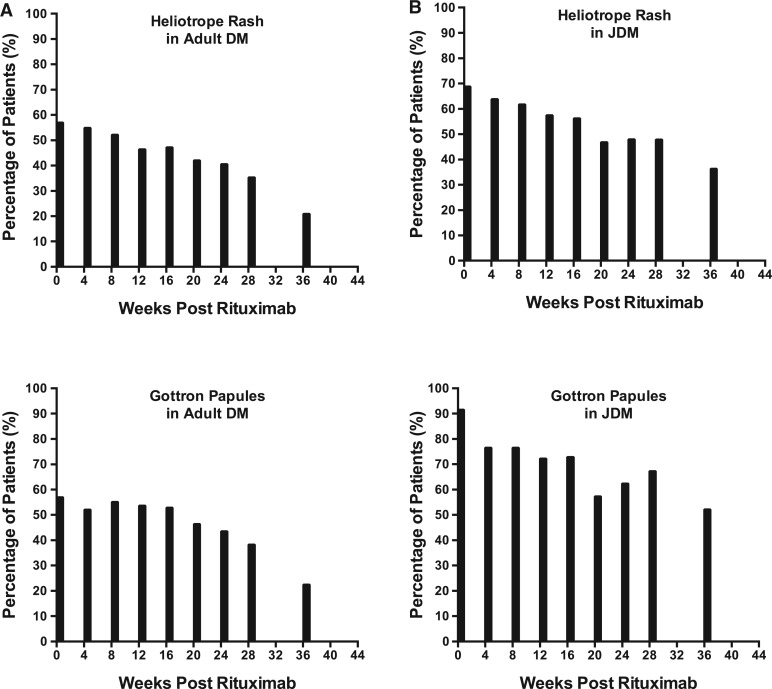
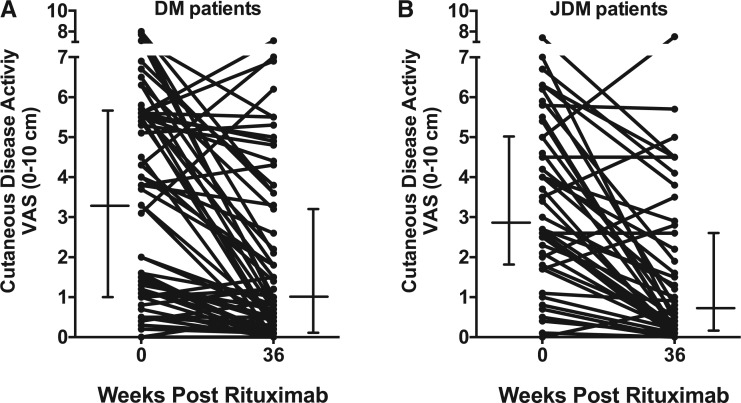
Overall, there was a significant improvement in the cutaneous disease activity after rituximab therapy, because the baseline to follow-up (week 36) VAS [mean (s.d.)] improved from 3.22 (2.46) to 1.72 (2.09), P = 0.0002 [median (interquartile range): from 3.3 (1–5.7) to 0.8 (0.1–3.2), P < 0.001]. The frequency of any DM rash decreased from 89% (64/72) at baseline to 76% (51/67) at week 36, P = 0.047 (five DM patients had missing MDAAT data at week 36). The frequency of classic DM rashes (heliotrope or Gottron changes) decreased from 69% (50/72) to 48% (32/67), P = 0.009. Among other individual rashes, a significant decrease in frequency was seen in erythroderma, erythematous rash without secondary changes of ulceration or necrosis, heliotrope, Gottron sign/papules, periungal erythema, diffuse alopecia and mechanics hands (Table 2). However, there was no significant improvement in cutaneous ulceration, panniculitis, erythematous rash with ulceration or necrosis or focal alopecia. Figure 1 graphically demonstrates this steady decline in the frequency of classic DM rashes after rituximab. A similar decline was seen in all of the other rashes that demonstrated a significant decrease in frequency (graphs not shown). The cutaneous disease activity score improved in most patients [67% (45/67)] by ⩾0.5 cm on the 10 cm VAS, while 21% (14/67) were stable and 12% (8/67) worse (Fig. 2). Among the damage variables, no major changes were seen in calcinosis, alopecia, cutaneous scarring or atrophy, poikiloderma and lipodystrophy (Table 2). The mean (s.d.) VAS score on MDI was 1.49 (2.02) at baseline and significantly decreased to 0.96 (1.53) at the week 44 time point, P = 0.003. The median time to improvement of rash by 40% was week 16. There was no significant difference in the time to improvement among various DM autoantibody subsets or by DM autoantibody-positive patients compared with autoantibody-negative patients.
Fig. 1.
Frequency of Gottron papules/sign and heliotrope in DM (A) and JDM (B)
Fig. 2.
Cutaneous disease activity at baseline and 36 weeks post-rituximab in DM patients (A) and JDM patients (B)
Effect of rituximab on cutaneous disease in adult DM
Patients in the rituximab early group demonstrated a trend for 20% relative improvement in their cutaneous disease activity score, faster than those in the rituximab late cohort (P < 0.052; Supplementary Fig. 1). Likewise, the rituximab early group also showed a trend for a more rapid improvement of 40 and 60% in cutaneous disease activity than the rituximab late group (P = 0.18 and 0.12, respectively).
Juvenile DM
Baseline disease activity and damage in JDM
Among the JDM subset, all patients had an active rash (48/48), which included the classic features of Gottron and/or heliotrope seen in 96% (46/48) patients. The baseline mean (s.d.) cutaneous disease activity VAS was 3.27 (2.04), with the most common individual rashes being Gottron papules in 92% (44/48) followed by erythematous rashes without erosion or necrosis in 83% (40/48) and heliotrope rash in 69%. Further details of the frequency of other JDM rashes at baseline and follow-up are given in Table 2. The baseline mean VAS (s.d.) cutaneous damage was 2.13 (2.34), with 46% of subjects having calcinosis, 23% with alopecia, 52% cutaneous scarring or atrophy, 23% poikiloderma and 13% with lipodystrophy.
Improvement in cutaneous rashes in JDM
There were significant improvements in the cutaneous disease activity, because the mean (s.d.) VAS reduced from 3.26 (2.04) at baseline to 1.56 (1.89) at week 36 (P < 0.0001) [median (interquartile range): from 2.9 (1.8–5.0) to 0.7 (0.2–2.7), P < 0.001]. The frequency of any JDM rash decreased from 100% (48/48) at baseline to 82% (36/44) at week 36 (P = 0.002). Likewise, the frequency of the classic JDM rashes (heliotrope or Gottron changes) decreased from 96% (46/48) to 64% (28/44) by study conclusion (P < 0.0001). Among individual rashes, a significant decrease was seen in cutaneous ulcerations, erythroderma, erythematous rash without ulceration or necrosis, heliotrope, Gottron sign/papules, periungual capillary changes and focal alopecia (Table 2). However, there was no significant improvement in panniculitis, erythematous rash with secondary changes of ulceration or necrosis, diffuse alopecia or mechanics hands. Figure 1 again graphically demonstrates the steady decline after rituximab in the frequency of the classic JDM rashes (heliotrope, Gottron papule/sign). A similar decline was seen in all other rashes that also significantly decreased (figure not shown). The cutaneous disease activity VAS score improved in most patients [75% (33/44) by ⩾0.5], while it was stable in 14% (6/44) and worse in 11% (5/44) (Fig. 2). Among the damage variables, no major changes were seen in calcinosis, alopecia, cutaneous scarring or atrophy, poikiloderma and lipodystrophy (Table 2). Likewise, there was no statistical improvement in the VAS MDI, because it was 2.13 (2.34) at baseline and 1.98 (2.27) at the week 44 time point (P = 0.47). The median time to improvement of the rash by 40% occurred by week 12. There was no significant difference in time to improvement among various JDM autoantibody subsets or by JDM autoantibody-positive patients compared with autoantibody-negative patients.
Effect of rituximab on cutaneous disease in JDM
Patients in the rituximab early arm did not show a trend for 20% improvement in their cutaneous disease activity score faster than patients in the rituximab late arm (P = 0.5, Supplementary Fig. 1), but showed a weak trend for 40 and 60% relative improvement (P = 0.22 and P = 11, respectively).
Discussion
The RIM Trial was the first prospective, randomized, double-blind trial in myositis, the largest clinical trial ever performed in the inflammatory myopathies, and the first to combine adult and paediatric idiopathic inflammatory myopathy subjects [8]. We sought to assess the efficacy of rituximab on the cutaneous features of adult DM and JDM in a refractory cohort of myositis patients and used validated measures of disease activity and damage in adult and paediatric myositis patients [9–11, 13]. Our results showed significant overall improvement in cutaneous disease activity in adult DM and JDM patients after rituximab. Moreover, many rashes, such as Gottron changes, heliotrope and other erythematous rashes, improved in DM and JDM patients, including treatment-resistant cutaneous ulceration in the JDM subset. In adult DM, the cohort of subjects receiving rituximab early also demonstrated a trend for faster improvement in cutaneous disease activity compared with those receiving rituximab late, suggesting a possible rituximab-specific effect on cutaneous improvement. But in the JDM cohort, there was only a weak trend toward a faster improvement at 40 and 60% in those subjects receiving rituximab early, but none at 20% improvement. However, it is important to note that, collectively, the RIM Trial patients represent a cohort with generally refractory myositis, in whom therapy with glucocorticoids and, on average, less than two additional immunosuppressive agents had failed over the course of their disease. Autoantibody subgroups did not show a significant difference in terms of cutaneous improvement, but this could be attributable to small numbers of patients in each autoantibody subgroup. It is also of note that no major worsening in cumulative cutaneous damage resulted over the 44 week course of the RIM Trial.
The pathogenesis of cutaneous disease in DM is not completely understood. Certainly, humoral immunity is implicated, with activation of the complement system leading to deposition of membrane attack complex in the capillaries. This leads to inflammation and necrosis of endothelial cells, thereby allowing activated T cells to migrate into muscle, causing ischaemia [16, 17]. It is possible that a similar mechanism is active in cutaneous manifestations of DM. Previous studies have shown more perivascular B lymphocytes than endomysial T lymphocytes in DM muscle, while skin lesions in DM show a predominance of T lymphocytes [18]. DM patients have also been found to have high serum concentrations of B-cell-activating factor, suggesting a more central role of the B cell [19, 20]. The characteristic features of a B cell antigen-driven autoimmune response in inflammatory myopathies include clonal expansion, class-switched somatic mutation and plasma cell maturation. In addition, B cells function as antigen presenting cells, co-stimulate T cells and secrete pro-inflammatory cytokines, thus making them a reasonable target in adult DM and JDM. Finally, the identification of several different autoantibodies in adult DM and JDM further supports the role of humoral immunity. Thus, our results demonstrate a significant improvement in the recalcitrant cutaneous lesions in DM after B cell depletion, supporting the role of the B cell in the pathogenesis of the cutaneous features of DM and JDM.
A potential limitation of the present study includes the post hoc nature of the analysis. However, we performed and presented results of all types of analyses that were performed, irrespective of results. Moreover, the analysis was driven by clinical questions relevant to the management of patients with DM rather than data mining. Another limitation inherent in the design of the RIM Trial is the difficulty in elucidating a true effect of rituximab compared with placebo, given the fact that both groups received rituximab within 8 weeks of each other. We attempted to overcome this limitation by assessing the time to 20, 40 and 60% improvement in the DM and JDM cohorts between the early vs late rituximab cohorts, but a lack of adequate power for subset analysis owing to smaller sample sizes limited the likelihood of finding a true effect, as well as a relatively short time difference (i.e. 8 weeks) between those subjects receiving rituximab early vs those receiving it later.
In conclusion, the refractory cutaneous rashes of DM and JDM improved in frequency as well as severity after rituximab in a large group of patients from the RIM Trial. However, it is difficult conclusively to attribute the efficacy directly to rituximab even though trends for more rapid improvement were observed in subjects receiving rituximab early compared with late.
Supplementary Material
Acknowledgements
We would like to thank all of the subjects and the participating centres and investigators who were involved in the RIM Trial as well as Genentech and the National Institutes of Health for their support of the RIM clinical trial.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: C.V.O. has received grant and clinical trial support from Genentech. R.A. has received research grants from Pfizer, Mallinckrodt and Genentech and has consulted for Novartis, Octapharma, Momenta and Bristol Myers-Squibb. All other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1. Cooper MA, Willingham DL, Brown DE. et al. Rituximab for the treatment of juvenile dermatomyositis: a report of four pediatric patients. Arthritis Rheum 2007;56:3107–11. [DOI] [PubMed] [Google Scholar]
- 2. Dinh HV, McCormack C, Hall S. et al. Rituximab for the treatment of the skin manifestations of dermatomyositis: a report of 3 cases. J Am Acad Dermatol 2007;56:148–53. [DOI] [PubMed] [Google Scholar]
- 3. Holzer U, van Royen-Kerkhof A, van der Torre P. et al. Successful autologous stem cell transplantation in two patients with juvenile dermatomyositis. Scand J Rheumatol 2010;39:88–92. [DOI] [PubMed] [Google Scholar]
- 4. Levine TD. Rituximab in the treatment of dermatomyositis: an open-label pilot study. Arthritis Rheum 2005;52:601–7. [DOI] [PubMed] [Google Scholar]
- 5. Tzaribachev N, Koetter I, Kuemmerle-Deschner JB. et al. Rituximab for the treatment of refractory pediatric autoimmune diseases: a case series. Cases J 2009;2:6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chung L, Genovese MC, Fiorentino DF. A pilot trial of rituximab in the treatment of patients with dermatomyositis. Arch Dermatol 2007;143:763–7. [DOI] [PubMed] [Google Scholar]
- 7. Chiu YE, Co DO. Juvenile dermatomyositis: immunopathogenesis, role of myositis-specific autoantibodies, and review of rituximab use. Pediatr Dermatol 2011;28:357–67. [DOI] [PubMed] [Google Scholar]
- 8. Oddis CV, Reed AM, Aggarwal R. et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum 2013;65:314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sultan SM, Allen E, Oddis CV. et al. Reliability and validity of the myositis disease activity assessment tool. Arthritis Rheum 2008;58:3593–9. [DOI] [PubMed] [Google Scholar]
- 10. Isenberg DA, Allen E, Farewell V. et al. International consensus outcome measures for patients with idiopathic inflammatory myopathies. Development and initial validation of myositis activity and damage indices in patients with adult onset disease. Rheumatology 2004;43:49–54. [DOI] [PubMed] [Google Scholar]
- 11. Pilkington C, Murray K, Isenberg I. et al. Development of disease activity and damage indices for myositis. Initial testing of four tools in juvenile dermatomyositis. Arthritis Rheum 2001;44:S294. [Google Scholar]
- 12. Rider L, Schiffenbauer A, Villalba M. et al. Extramuscular disease activity is frequent in adult and juvenile idiopathic inflammatory myopathies (IIM) and does not correlate with other myositis activity measures. Arthritis Rheum 2002;46:S612–3. [Google Scholar]
- 13. Sultan SM, Allen E, Cooper K. et al. Inter-rater reliability of two disease activity and damage assessment tools in patients with idiopathic inflammatory myopathy—report of 105 patients. Arthritis Rheum 2004;50:S668. [Google Scholar]
- 14. Rider LG, Giannini EH, Brunner HI. et al. International consensus on preliminary definitions of improvement in adult and juvenile myositis. Arthritis Rheum 2004;50:2281–90. [DOI] [PubMed] [Google Scholar]
- 15. http://www.niehs.nih.gov/research/resources/imacs/diseaseactivity.
- 16. Khanna S, Reed AM. Immunopathogenesis of juvenile dermatomyositis. Muscle Nerve 2010;41:581–92. [DOI] [PubMed] [Google Scholar]
- 17. Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet 2003;362:971–82. [DOI] [PubMed] [Google Scholar]
- 18. Santmyire-Rosenberger B, Dugan EM. Skin involvement in dermatomyositis. Curr Opin Rheumatol 2003;15:714–22. [DOI] [PubMed] [Google Scholar]
- 19. Krystufková O, Vallerskog T, Helmers SB. et al. Increased serum levels of B cell activating factor (BAFF) in subsets of patients with idiopathic inflammatory myopathies. Ann Rheum Dis 2009;68:836–43. [DOI] [PubMed] [Google Scholar]
- 20. Baek A, Park HJ, Na SJ. et al. The expression of BAFF in the muscles of patients with dermatomyositis. J Neuroimmunol 2012;249:96–100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.