Abstract
Anti-Flavivirus antibodies are highly cross-reactive and may facilitate Zika virus (ZIKV) infection through the antibody-dependent enhancement (ADE) mechanism. We demonstrate that dengue-specific antibodies enhance the infection of a primary Brazilian ZIKV isolate in a FcγRII-expressing K562 cell line. In addition, we demonstrate that serum samples from dengue-immune pregnant women enhanced ZIKV infection. These findings highlight the need for epidemiological studies and animal models to further confirm the role of ADE in the development of congenital and neurological complications associated with ZIKV infections.
Keywords: Zika virus, antibody dependent enhancement, dengue antibodies.
Zika virus (ZIKV) is currently in the spotlight as a major public health concern worldwide [1]. Until recently, ZIKV infections in humans were associated with either asymptomatic or self-limiting exanthematic illness. However, the emergence of ZIKV in Polynesia and Brazil revealed the virus is associated with more severe clinical manifestations than previously reported, including neurological complications in adults (eg, Guillain-Barré Syndrome) and microcephaly in newborns [2–4].
The factors responsible for the large variability of severe phenotypes [3] of ZIKV infection remain unclear. Dengue virus (DENV) antibodies have been shown to enhance ZIKV infection [5, 6]. These antibodies cross-react with ZIKV structural proteins, facilitating virus uptake by cells expressing FcγR receptors through the mechanism of antibody-dependent enhancement (ADE) [5, 6]. Because of the high rate of dengue transmission in Brazil, >90% of the adult population has been previously exposed to DENV, especially in the Northeast region [7]. Here, we used a panel of serum samples from individuals with different dengue immunity profiles to confirm that dengue antibodies enhance the infection of a Brazilian ZIKV isolate.
MATERIALS AND METHODS
Sera Panel
We included well-characterized serum samples of (1) 6 laboratory-confirmed symptomatic dengue infections (primary and secondary dengue cases), (2) 20 pregnant women with different dengue immunity profiles, and (3) 3 dengue-naive pregnant women.
Dengue symptomatic patient serum samples were collected from individuals enrolled in a cohort of dengue suspected cases conducted in the city of Recife [8], a large urban center and hyperendemic area of dengue in Northeast Brazil. Sequential blood samples were collected from each patient during the acute (<7 days) and convalescent phases (10–15 days) as well as around 30 days after enrollment in the study. Additional samples were obtained 6 and 12 months later. Dengue cases were laboratory confirmed by the combination of virus isolation in C6/36 cells and viral RNA detection by reverse-transcriptase polymerase chain reaction (RT-PCR) with dengue serology for immunoglobulin M (IgM)/immunoglobulin G (IgG) by enzyme-linked immunosorbent assay (ELISA). The kinetics of IgM and IgG response were used to classify the cases as primary or secondary dengue infections. For this study, we included serum samples from primary (n = 3) and secondary dengue cases (n = 3) collected at the following time points: <7 days, 10–15 days, 30–40 days, and >260 days after infection [8].
Serum samples were obtained from healthy pregnant women included in a prospective dengue birth cohort study carried out in the same setting [9, 10]. These samples were collected between 2011 and 2012 and represent the dengue epidemiological profile of women at reproductive age shortly before the circulation of ZIKV in this area. Dengue serological profiles of the mothers included in the cohort were determined by ELISA and plaque reduction neutralization test (PRNT) [9, 10]. To investigate ADE of ZIKV infection by DENV antibodies, 2 groups plus a control group of mothers were selected based on their PRNT status: Group I was comprised of serum samples from mothers with a monotypic DENV-immune profile (PRNT50 > 20 to only 1 serotype; n = 10); and Group II included sera from mothers with multitypic immunity (PRNT50 > 20 to >1 DENV serotype; n = 10) (Supplementary Table 2). Serum samples from DENV-naive mothers enrolled in the cohort were also included in this study (n = 3). Samples were confirmed as DENV-negative by IgM/IgG serology and PRNT. Details of the study design and data collection of both cohort studies have been previously described [8, 9]. A detailed description of the serological characterization of each sera included in this study has been provided in Supplementary Tables 1 and 2.
Viruses
Zika virus PE/243 and DENV-2 16681 (prototype strain) were used in the ADE assay. Zika virus PE/243 was isolated from a Zika case diagnosed in Pernambuco state, Northeast Brazil [11]. The virus strains were propagated in Vero cells, as described elsewhere [10]. The virus-containing supernatants were harvested from infected Vero cells and clarified by centrifugation (930 g, 10 minutes, 4°C). Virus particles were precipitated with 50% polyethylene glycol (PEG 3100) in Dulbecco’s modified Eagle medium (DMEM). Briefly, the supernatant/PEG mixture (proportion of 1:4) was incubated overnight at 4°C. After centrifugation (1500 g, 30 minutes, 4°C), the supernatant was discarded, and the pellet containing the virus particles was resuspended in one hundredth of the original volume of supernatant/PEG in DMEM with 25 mM of HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid). Zika virus PE/243 was titrated by plaque assay, whereas DENV-2 16681 virus titer was determined by focus-forming assay [10].
Antibody-Dependent Enhancement Assay
FcγRII-expressing K562 cell line were exposed to ZIKV PE/243 in the presence of either a Flavivirus-naive (AB human serum) or a dengue-immune serum sample (DENV-3 immune serum). Antibody-dependent enhancement was determined by flow cytometry, as previously described [10]. Antibody-dependent enhancement was measured as the n-fold increase in the percentage of virus-infected cells relative to that in a Flavivirus-naive serum. DENV-2 16681 was used as a control for the assay.
Quantitative Real-Time Polymerase Chain Reaction
Zika virus RNA was extracted from culture supernatants using QIAamp Viral RNA extraction kit following the manufacture’s specifications. Quantitative RT-PCR was conducted by using the QuantiTect Probe RT-PCR Kit with amplification in the Applied Biosystems 7500 real-time PCR system following the manufacturer’s protocol. Zika virus primers (ENV1086F:5’-CCGCTGCCCAACACAAG-3’ and ENV1162R:5’-CCACT AACGTTCTTTTGCAGACAT-3’) and probe (5’-VIC AGCCTA CCTTGACAAGCAGTCAGACACTCAA-BHQ1-3) sequences for the quantitative virus detection assay were designed according to Lanciotti et al (2008) [12]. The relative quantification of ZIKV RNA was assessed using the 7500 Software v2.0.6.
Ethical Statement
The protocol was approved by the Ethical Committee of Aggeu Magalhaes Research Center (CAAE-0061.0.095.000-10) and of the Brazilian Ministry of Health (CONEP 25000.119007/2002–03; CEP68/02).
Statistical Analysis
Student’s t test was used to compare percentage of infected cells and enhancing activity between 2 groups. The correlation between previous dengue immunity and ADE was determined using the Spearman test. The level of significance was set at .05. Statistical analysis was performed using Graph Pad Prism, version 6.0e.
RESULTS
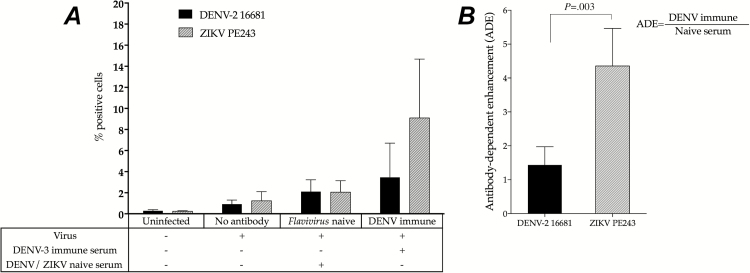
We postulated that nonneutralizing, cross-reactive, dengue-specific antibodies enhance ZIKV infection of FcγR receptor-bearing phagocytes through the mechanism of ADE. To confirm that, FcγRII-expressing K562 cell lines were exposed to the local Brazilian ZIKV isolate PE/243 in the presence of either a Flavivirus-naive or a dengue-immune serum sample. Zika virus and DENV-2 infectivity were low on K562 cells infected without antibodies (1.23% ± 0.87% and 0.91% ± 0.39%, respectively) or in the presence of Flavivirus-naive serum (2.05% ± 1.09% and 2.11% ± 1.11%, respectively). However, preincubation of ZIKV with a serum sample from a DENV-3–immune individual increased the infectivity of both viruses compared with the Flavivirus-naive sample (9.09% ± 5.58% for ZIKV and 3.45% ± 3.20% for DENV-2). The ADE observed for ZIKV was 3 times greater than the one observed for DENV-2 (4.35 ± 1.11 and 1.43 ± 0.53, respectively; P = .003). The experiment was independently performed 4 times on different days to assure reproducibility. A representative analysis is shown in Figure 1A and 1B.
Figure 1.
Antibody-dependent enhancement (ADE) of Zika virus (ZIKV) infection by dengue-specific antibodies. FcγRII-expressing K562 cells were infected with ZIKV PE/243 in the absence of antibodies or in the presence of either a Flavivirus-naive serum or a dengue virus (DENV)–3 immune serum. Antibody-dependent enhancement was measured as the n-fold increase in the percentage of virus-infected cells relative to that in a Flavivirus-naive serum. A, Percentage of DENV- and ZIKV-infected K562 cells in absence or in presence of serum. B, Antibody-dependent enhancement of ZIKV in K562 cells.
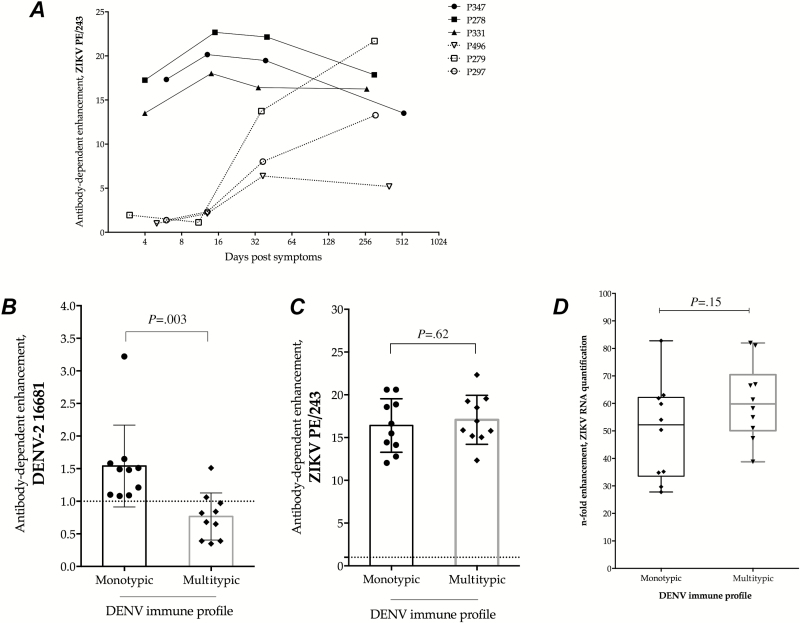
Next, we determined the kinetics of ADE of ZIKV infection in a panel of well-characterized serum samples from primary (n = 3) and secondary (n = 3) laboratory-confirmed dengue cases collected from acute (<7 days after onset of symptoms) and convalescent (10–15 days after onset of symptoms) phases until after complete recover (30–40 days and >260 days after onset of symptoms). Antibody-dependent enhancement of ZIKV infection was not observed in samples collected during the febrile and acute phases (before serum conversion) in patients experiencing dengue primary infections (Figure 2A). Antibody-dependent enhancement was only observed in this group at later time points, after convalescence and recovery (Figure 2A). In contrast, serum samples from dengue secondary cases induced ADE of ZIKV infection regardless of the phase of infection analyzed (Figure 2A).
Figure 2.
Antibody-dependent enhancement (ADE) of Zika virus (ZIKV) infection by dengue antibodies. A, Longitudinal serum samples from dengue-infected patients were used to determine the kinetics of ADE of ZIKV infection after dengue exposure in individuals experiencing a primary (dotted lines) or secondary (solid lines) dengue infection. Antibody-dependent enhancement of dengue virus (DENV) (B) and ZIKV (C) was tested in the presence of a panel of serum samples from pregnant women with different dengue immune status, as determined by plaque reduction neutralization test: monotypic (DENV-3) (n = 10) and multitypic (DENV-3 and DENV-4) (n = 10). C, The n-fold increase in the ZIKV RNA quantification in the presence of monotypic or multitypic dengue immune serum relative to that in a Flavivirus-naive serum. Abbreviations: ADE, Antibody-dependent enhancement; DENV, dengue virus; ZIKV, Zika virus, vRNA, virus ribonucleic acid.
We then selected a panel of serum samples from pregnant women immune to either DENV-3 alone (monotypic; n = 10) or in combination with DENV-4 (multitypic; n = 10), as determined by PRNT assays. All samples from DENV-immune mothers show ADE of ZIKV infection (n = 20/20), whereas ADE was not observed among the DENV-naive mothers (n = 0/3). Serum samples from monotypic and multitypic dengue-immune mothers induced greater ADE of ZIKV infection (16.41 ± 3.12 and 17.08 ± 2.86, for monotypic and multitypic, respectively, as compared with DENV-2 (1.54 ± 0.62 and 0.76 ± 0.36, for monotypic and multitypic, respectively) (Figure 2C and 2B). However, immunity to multiple DENV serotypes inhibited the infectivity of DENV-2 (P = .003; Figure 2B), whereas ADE of ZIKV was elevated in monotypic and multitypic groups (P = .62) (Figure 2C).
Antibody-dependence enhancement of ZIKV infection by dengue-specific antibodies was also observed after quantification of viral RNA by quantitative RT-PCR in culture supernatants of infected K562 cells. Viral RNA levels increased up to 7-fold in the presence of dengue immune serum compared with the Flavivirus-naive serum (Figure 2D). There was no difference in the ZIKV RNA levels collected from supernatants harvested from infected cells in the presence of dengue monotypic or multitypic maternal sera (P = .15).
DISCUSSION
Although ZIKV has been linked to the increased incidence of congenital microcephaly cases [3, 4], the mechanisms underlying ZIKV transmission from mother to fetus remain unknown. Here, we demonstrated that the presence of dengue antibodies increased the infectivity of a primary Brazilian ZIKV isolate in a human cell line expressing FcγRII receptors.
Antibody-dependent enhancement of ZIKV infection in mononuclear phagocytes was first evidenced in the 1980s [13] and has been recently confirmed by others [5, 6]. Dejnirattisai et al [5] showed that pooled convalescent serum and monoclonal antibodies derived from DENV-infected patients were able to promote ADE of ZIKV infection on the monocyte cell line U937 [5]. Our results confirmed these findings in a different cell line and also explored the kinetics of ADE on paired samples taken from the same subjects at different time points, contributing to better understanding of how preexisting Flavivirus immunity influences ZIKV infection in vitro. We acknowledge that it is not possible to definitively rule out previous ZIKV exposure of the patients included in our study, although there were no reports of ZIKV or microcephaly outbreaks in Brazil between 2004–2006 and 2011–2012, when the samples were collected. Of note, monoclonal antibodies directed to the envelope dimer epitope of DENV have been recently demonstrated to potently neutralize ZIKV infection and inhibit ADE in vitro [5, 14], pointing to a potential immunotherapy against ZIKV and opening the venues for the identification of ZIKV and DENV shared epitopes able to elicit neutralizing antibodies against both viruses [14].
Interestingly, we showed that sera from pregnant women—representative of the pregnant population of Recife, which was the epicenter of the microcephaly epidemic in Brazil— promote ADE of ZIKV infection in vitro. Noteworthy, increased numbers of congenital disease associated with ZIKV infection have not been reported in Southeast Asia countries [15], where dengue has been hyperendemic for >5 decades and pregnant women are usually immune to all DENV serotypes. Unlike Asia, pregnant women from our setting are mostly immune to DENV-3 alone and probably have lower levels of DENV-associated antibodies than women exposed to several DENV infections [10]. Thus, we cannot exclude the possibility that background immunity and DENV-specific antibody levels of the population might contribute to increased disease severity of ZIKV infections. This probably explains the higher rates of congenital syndrome and neurological complications associated with ZIKV infections observed in Brazil compared with Asian countries [4, 15], although virus virulence of the circulating ZIKV might also play a role.
In summary, we demonstrated that dengue-specific antibodies dramatically increase ZIKV infectivity in vitro in phagocytes expressing FcγRII receptors. This finding might have implications for the immunopathogenesis of ZIKV infection in dengue-endemic areas. We acknowledge that the influence of ADE in determining severe disease in vivo has been controversial in the dengue field [10]. To date, there has been no epidemiologic evidence of enhanced ZIKV illness during the ongoing epidemic in South and Central America. Although our data clearly demonstrate the ability of dengue antibodies to enhance ZIKV infection in vitro, it is important to point out that the relevance of this mechanism in vivo must be carefully explored. Additional epidemiological and animal model studies are needed to elucidate the contribution of previous dengue immunity in mediating congenital and neurological complications associated with ZIKV infections.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank Dr Renato Oliveira and Ms Verônica Gomes (Aggeu Magalhães Research Center) for reagents, help with cytometer, and samples selection. We also thank Dr Donald Burke for advice and invaluable discussion.
Financial support. This work was supported by the Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES); Center for Vaccine Research, University of Pittsburgh; Fogarty Training Program (D43TW006592 Pitt GIDRTP/ 323 NIH to P. M. S. C.); National Council for Scientific and Technological Development (CNPq) (482915/2010-2 MCT/CNPq-321 14/2010); Strategic Program to Support Health Research/ PAPES VI (322 407697/2012-8); and the National Institute of Allergy and Infectious Diseases (U19 AI56541).
Potential conflicts of interest. All authors. No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lessler J, Chaisson LH, Kucirka LM, et al. Assessing the global threat from Zika virus. Science 2016; 353:aaf8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cao-Lormeau VM, Blake A, Mons S, et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 2016; 387:1531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moore CA, Staples JE, Dobyns WB, et al. Characterizing the pattern of anomalies in congenital zika syndrome for pediatric clinicians. JAMA Pediatr 2016 E1–8. doi:10.1001/jamapediatrics.2016.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Araújo TV, Rodrigues LC, de Alencar Ximenes RA, et al. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: preliminary report of a case-control study. Lancet Infect Dis. 2016; 16:1356–63. [DOI] [PubMed] [Google Scholar]
- 5. Dejnirattisai W, Supasa P, Wongwiwat W, et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with Zika virus. Nat Immunol 2016; 17:1102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Priyamvada L1, Quicke KM1, Hudson WH, et al. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc Natl Acad Sci U S A 2016; 113:7852–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Braga C, Luna CF, Martelli CM, et al. Seroprevalence and risk factors for dengue infection in socio-economically distinct areas of Recife, Brazil. Acta Trop 2010; 113:234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cordeiro MT, Silva AM, Brito CA, et al. Characterization of a dengue patient cohort in Recife, Brazil. Am J Trop Med Hyg 2007; 77:1128–34. [PubMed] [Google Scholar]
- 9. Braga C, Albuquerque MFPM, Cordeiro MT, et al. Prospective birth cohort in a hyperendemic dengue area in Northeast Brazil: methods and preliminary results. Cad Saude Publica 2016; 32 :1–11. [DOI] [PubMed] [Google Scholar]
- 10. Castanha PMS, Braga C, Cordeiro MT, et al. Placental transfer of dengue-specific antibodies and kinetics of dengue infection enhancing-activity in Brazilian infants. J Infect Dis 2016; 214:265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Donald CL, Brennan B, Cumberworth SL, et al. Full genome sequence and sfRNA interferon antagonist activity of Zika virus from Recife, Brazil. PLoS Negl Trop Dis 2016; 10:e0005048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008; 14: 1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fagbami AH, Halstead SB, Marchette NJ, Larsen K. Cross-infection enhancement among African flaviviruses by immune mouse ascitic fluids. Cytobios 1987; 49:49–55. [PubMed] [Google Scholar]
- 14. Swanstrom JA, Plante JA, Plante KS, et al. Dengue virus envelope dimer epitope monoclonal antibodies isolated from dengue patients are protective against Zika virus. MBio 2016; 19 :1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Durbin AP. Dengue antibody and Zika: friend or foe? Trends Immunol 2016; 37:635–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.