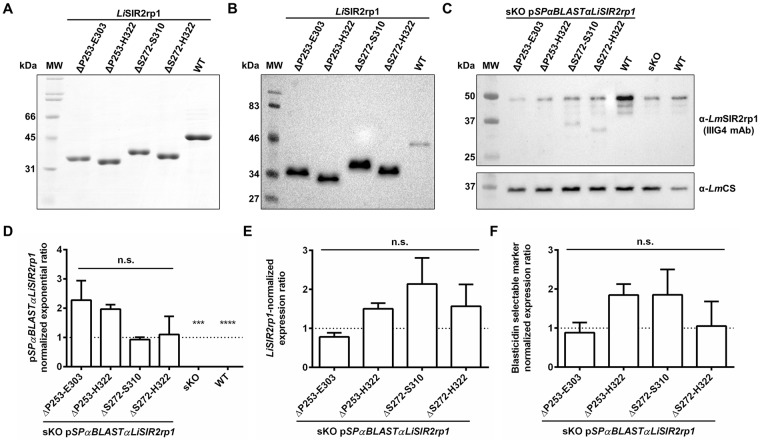
Fig 6. Expression of LiSIR2rp1 truncated forms in promastigotes.
(A) Coomassie blue-stained SDS-PAGE gel of 5 μg of recombinant LiSIR2rp1 protein (WT) and respective truncated forms (ΔP253-E303; ΔP253-H322; ΔS272-S310; ΔS272-H322). (B) Western blot showing recognition of all LiSIR2rp1 recombinant forms by anti-L. major SIR2rp1 (α-LmSIR2rp1) IIIG4 monoclonal antibody (mAb). (C) Western blot analysis of LiSIR2rp1 levels in LiSIR2rp1 single knockout (sKO) promastigotes complemented with each pSPαBLASTαLiSIR2rp1 construct, as well as in sKO and WT parasites. Cysteine synthase (CS) was used as the loading control. (D) Quantification of pSPαBLASTαLiSIR2rp1 constructs by qPCR using genomic DNA of transfected, sKO and WT parasites, relative to sKO pSPαBLASTαLiSIR2rp1WT. (E, F) Expression levels of LiSIR2rp1 (E) and the blasticidin resistance marker (F) determined by qPCR using cDNA from transfected parasites, relative to sKO pSPαBLASTαLiSIR2rp1WT. Real time quantitative PCR (qPCR) analysis was performed using LirRNA45 as the reference gene. Means and standard deviations of two independent experiments are represented. Unpaired t-test with Welch’s correction was performed: ***, p < 0.01; ****, p < 0.0001; n.s., non-significant.