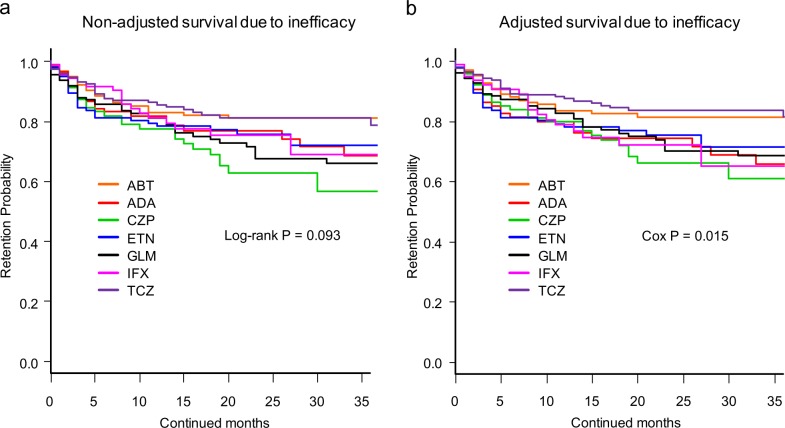
Fig 2.
Drug survival rates due to inefficacy of (a) non-adjusted and (b) adjusted cases. Adjusted confounder s were baseline sex, age, disease duration, DAS28-ESR, HAQ-DI, RF and ACPA positivity, concomitant MTX and PSL dose, presence of concomitant csDMARDs (BUC, IGU, SASP, and TAC), date of starting bDMARDs, and number of previously used bDMARDs. ABT = abatacept, ADA = adalimumab, CZP = certolizumab pegol, ETN = etanercept, GLM = golimumab, IFX = infliximab, TCZ = tocilizumab, DAS28-ESR = Disease Activity Score in 28 joints using erythrocyte sedimentation rate, HAQ-DI = Health Assessment Questionnaire disability index, RF = rheumatoid factor, ACPA = anti- cyclic citrullinated peptide antibody, MTX = methotrexate, PSL = prednisolone, csDMARDs = conventional synthetic disease-modifying antirheumatic drugs, BUC = bucillamine, IGU = iguratimod, SASP = salazosulfapyridine, TAC = tacrolimus, bDMARDs = biological disease-modifying antirheumatic drugs.