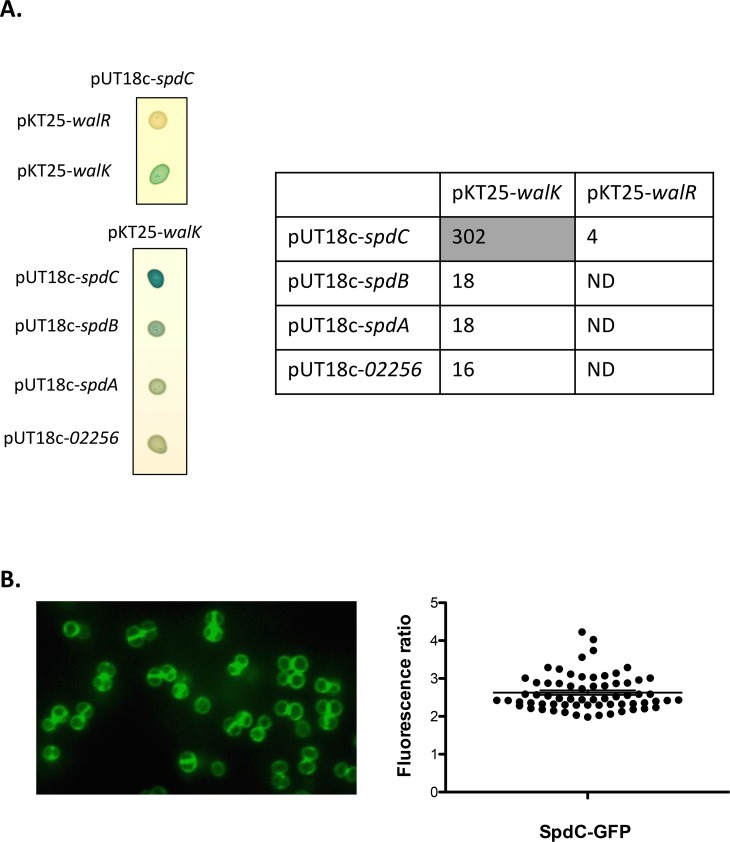
Fig 3. The SpdC membrane protein forms a complex with the WalK histidine kinase at the division septum.
A: The SpdC protein was tested for pairwise interactions with the WalK histidine kinase and the WalR response regulator using the BACTH complementation assay by translationally fusing the full length corresponding coding sequences with those of the T25 or T18 adenylate cyclase domains (upper left panel). Specificity of the SpdC-WalK complex was confirmed by testing interactions with three other S. aureus Abi-domain proteins: SpdB, SpdA and SAOUHSC_02256 (lower left panel). Cultures of E. coli DHT1 strains co-transformed with the indicated plasmid combinations were spotted on LB agar plates with X-Gal as an indicator of β-galactosidase activity (see Materials and Methods). Quantitative β-galactosidase activity assays for each strain are indicated in the right panel (expressed in Miller Units). The shaded cell indicates significant β-galactosidase activity resulting from positive protein-protein interactions. ND: Not determined. B: S. aureus HG001 cells producing a fluorescent SpdC-GFP protein fusion (HG001/pOLSA-spdC) were grown in TSB with 0.25 μM CdCl2 and observed in exponential phase by fluorescence microscopy. Fluorescence ratios (septum/lateral membrane) were quantified for two biological replicates (33 cells from each) using ImageJ software and plotted using GraphPad Prism. The horizontal line indicates the mean fluorescence ratio and the error bars represent the SEM.