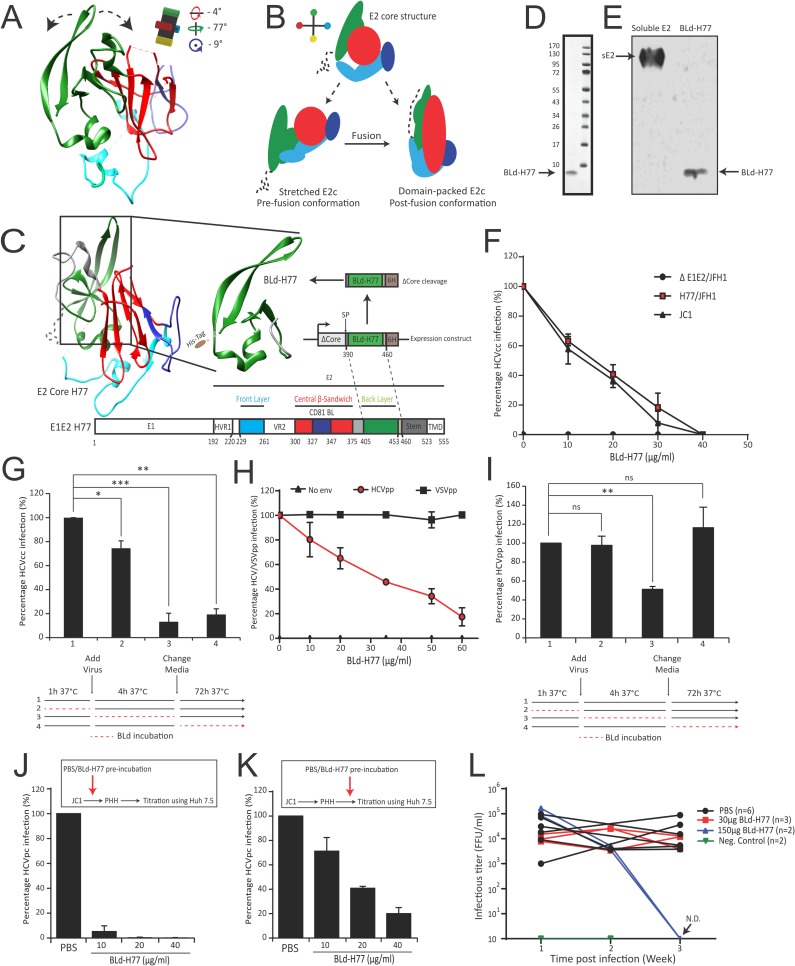
Fig 4. An E2 BLd-derived peptide inhibits HCV infection.
(A) Putative BL movements that can modulate E2 rearrangements within E1E2 heterodimers. Rotation angles of the E2core structure are indicated (Reference: Fig 3B) and illustrated by a black cross harboring 4 color extremities. (B) Schematic representation of the E2 core structure and of the two hypothetical E2 conformational states prior (stretched) and following (packed) virus fusion when E1E2 are associated as heterodimer (color code and angle of E2 structures refer to Fig 3B). (C) Schematic representation of the expression construct encoding for the BLd-H77 peptide derived from the gt1a H77 E2 glycoprotein. SP, signal peptide; 6H, 6His-tag. (D) Detection of BLd-H77 in non-reducing condition following SDS-Page electrophoresis and coomassie blue staining. Molecular weight of the reference ladder are indicated on the left (kDa). (E) Detection of BLd-H77 peptide by Western Blot using a 6His-tag antibody. A 6His-tagged soluble E2 was used as a positive control. (F) Dose-dependent inhibition of HCVcc infection. HCVcc H77/JFH-1 or JC1 were pre-incubated with different doses of BLd-H77 (μg/ml) and used to infect Huh7.5 cells. Percentages of primary infection were calculated according to the viral titers of HCVcc particles incubated without BLd-H77 (mean ± SD; n = 3). (G) Kinetic of action of BLd-H77 on HCVcc infection. Huh7.5 cells were incubated 1h with BLd-H77 prior infection (35μg/ml) of HCVcc H77/JFH-1 (2), during the 4h infection (3) or following infection (secondary infection; 4). As control, cells were incubated at each step with equivalent volume of PBS (1). Percentages of primary infection were calculated according to viral titers of control conditions (mean ± SD; n = 3). Statistical significances (*p<0.05, **p<0.01, ***p<0.001) were determined for each experimental condition versus control condition (100%). (H) Dose-dependent inhibition of HCVpp harboring H77 envelope (HCVpp-H77) or control pseudoparticles harboring VSV-G (VSVGpp) envelope glycoprotein. Pseudoparticles were pre-incubated with different doses of BLd-H77 (μg/ml) or with PBS and used to infect Huh7.5 cells. Percentage of GFP positive cells was determined and expressed as percentages of infection, according to the viral titers of HCVpp incubated with PBS (mean ± SD; n = 3). (I) Kinetic of action of BLd-H77 on HCVpp infection. Huh7.5 cells were incubated 1h with BLd-H77 prior HCVpp H77 infection (50μg/ml) (2), during the 4h infection (3) or following infection (4). As control, cells were incubated at each step with equivalent volume of PBS (1). Percentages of infection according to viral titers of control conditions are reported (mean ± SD; n = 3). Statistical significances (**p<0.01, ns non-significant) were determined for each experimental condition versus control condition (100%). (J) Dose-dependent inhibition of HCVcc JC1 virus infection of primary human hepatocytes (PHH). JC1 particles were pre-incubated with different doses of BLd-H77 (μg/ml) or with PBS and then used to infect PHH. PHH cell culture media were harvested four days post-infection and used to infect naïve Huh7.5 cells. Percentages of secondary infection are shown and calculated according to the viral titers of JC1 virus pre-incubated with PBS (mean ± SD; n = 3). (K) Dose-dependent inhibition of JC1-derived HCVpc (HCV primary cell culture-derived) of Huh7.5. HCVpc particles were pre-incubated with different doses of BLd-H77 (μg/ml) or with PBS and used to infect naïve Huh7.5 cells. Percentages of primary infection using HCVpc particles are shown and calculated according to the viral titers of JC1 virus pre-incubated with PBS (mean ± SD; n = 3). (L). Inhibition of HCV infection in vivo in humanized liver mice. JC1 infectious titers (FFU/ml) were obtained from two independent mice cohorts treated with 30μg (red, n = 3) or 150μg (blue, n = 2) of BLd-H77, or with PBS (black; cohort = 6) under a “prophylactic” protocol. BLd-H77 or PBS was injected one day prior virus infection with JC1 HCVcc particles and subsequent injections were performed at day 1, 7 and 14 post-infection. Sera were harvested 7 (Week 1), 14 (Week 2) and 21 days (Week 3) post-infection. HCV viral titers were determined through infection of Huh7.5 cells with mouse sera and quantification of FFU/ml. One non-engrafted liver mice and one non-infected mice were used as negative controls (green, n = 2). N.D., non-detected.